The Role of Pharmacokinetics in Generic Drugs
Drug Patent Watch
OCTOBER 7, 2024
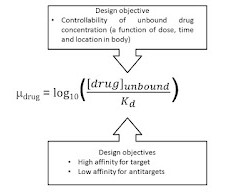
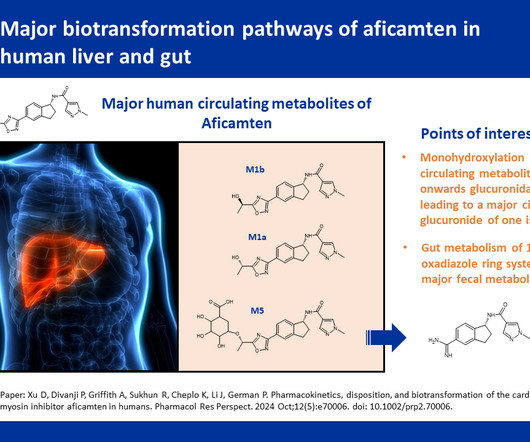
Pharmacokinetics (PK) plays a crucial role in the development and approval of generic drugs. PK studies help ensure that generic drugs are bioequivalent to their brand-name counterparts, meaning they have similar absorption, distribution, metabolism, and excretion (ADME) profiles.









































Let's personalize your content