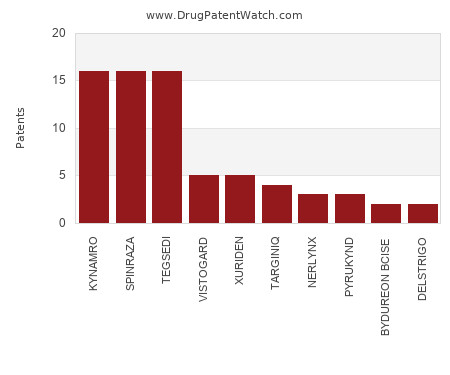
Which pharmaceutical drugs have the most drug patents in Finland?
Drug Patent Watch
FEBRUARY 4, 2024
This chart shows the drugs with the most patents in Finland. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is desired.… The post Which pharmaceutical drugs have the most drug patents in Finland? appeared first on DrugPatentWatch - Make Better Decisions.










Let's personalize your content