All eyes on Lab Innovations 2021
Drug Discovery Today
JULY 18, 2021
~ UK’s biggest trade show for the laboratory industry returns for 2021 ~

Drug Discovery Today
JULY 18, 2021
~ UK’s biggest trade show for the laboratory industry returns for 2021 ~

Eye on FDA
JULY 29, 2021
In the face of a crisis situation, it is a given that the clarity and thoroughness of the communications response is key to resolving the issue and mitigating any reputational damage. Perhaps no other decision by the Food and Drug Administration has garnered as much controversy, as the recent one to authorize the accelerated approval a new treatment for Alzheimer’s.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drug Patent Watch
JULY 16, 2021
Come join me and Wendi Lau from Abbvie as we participate in an interactive roundtable discussion on pharmaceutical portfolio management. We’ll be sharing our expertise and best practices for strategic…. The post DrugPatentWatch and Abbvie discuss Pharmaceutical Portfolio Management appeared first on DrugPatentWatch - Make Better Decisions.

Drug Channels
JULY 30, 2021
Today’s guest post comes from David Holladay, President of Access and Adherence at CoverMyMeds. David discusses how common medication access, adherence, and affordability barriers can lead patients to abandon important therapies. He suggests that technology can connect providers with actionable information at the point of care, thereby giving patients access to data that can lead them to make better decisions.

Speaker: Simran Kaur, Co-founder & CEO at Tattva.Health
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

The Pharma Data
JULY 29, 2021
econd Quarter 2021 Product Sales Increased 21% Year-Over-Year Primarily Driven by Veklury. Biktarvy Sales Increased 24% Year-Over-Year. Gilead Sciences, Inc. (Nasdaq: GILD) announced today its results of operations for the second quarter 2021. “We maintained our positive momentum in the second quarter, with both a solid financial performance and strong progress across our increasingly diverse portfolio.
Drug Discovery Digest brings together the best content for drug research and development professionals from the widest variety of industry thought leaders.

Drug Discovery Today
JULY 8, 2021
The topic of this month’s newsletter from Drug Discovery Today is “Cardiovascular Drugs”.

Eye on FDA
JULY 19, 2021
It has been a regular feature of the blog to give a read on what FDA has been talking about, at least through the form of press releases. It is not always an easy task because the nature of FDA’s practice in this respect has evolved over time. But here is what we see looking at the first 6 months 2021, and comparing it to mid-years past. You may recall from the last posting on this topic at the beginning of the year, during 2020, FDA had a lot to say – a real lot, and that isn’
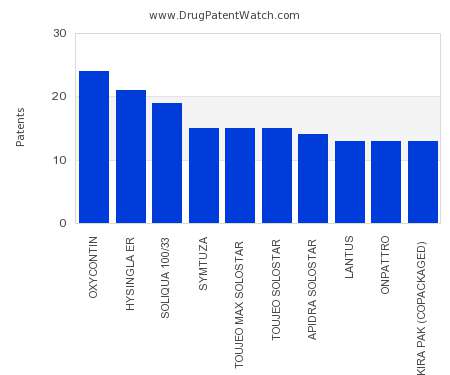
Drug Patent Watch
JULY 30, 2021
This chart shows the drugs with the most patents in Cyprus. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is desired.…. The post Which pharmaceutical drugs have the most drug patents in Cyprus? appeared first on DrugPatentWatch - Make Better Decisions.
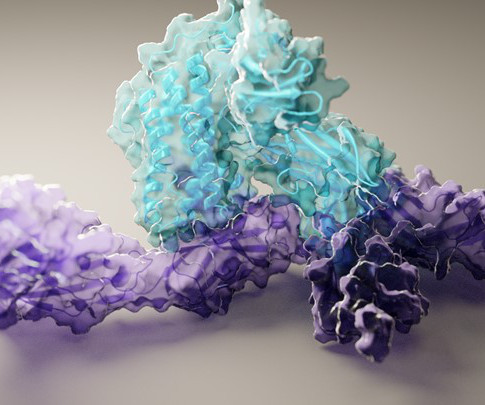
NIH Director's Blog: Drug Development
JULY 27, 2021
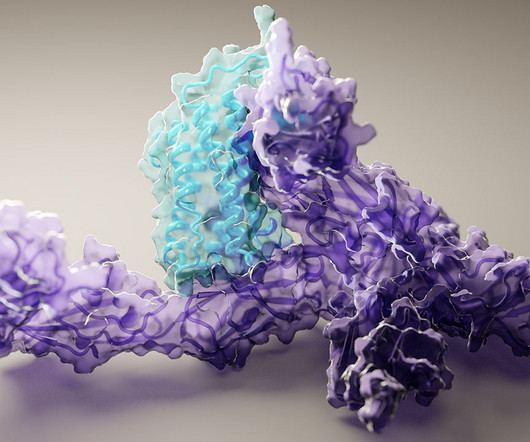
Caption: Researchers used artificial intelligence to map hundreds of new protein structures, including this 3D view of human interleukin-12 (blue) bound to its receptor (purple). Credit: Ian Haydon, University of Washington Institute for Protein Design, Seattle Proteins are the workhorses of the cell. Mapping the precise shapes of the most important of these workhorses helps to unlock their life-supporting functions or, in the case of disease, potential for dysfunction.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

The Pharma Data
JULY 29, 2021
Second-Quarter 2021 Revenues of $19.0 Billion, Reflecting 86% Operational Growth; Excluding BNT162b2(1) , Revenues Grew 10% Operationally to $11.1 Billion The 10% Operational Growth Excluding BNT162b2(1) in Second-Quarter 2021 Builds on the 6% Operational Growth Delivered by the Comparable Business in Second-Quarter 2020 Second-Quarter 2021 Reported Diluted EPS(2) of $0.98, Adjusted Diluted EPS(3) of $1.07 Raises Full-Year 2021 Guidance(4) for Revenues to a Range of $78.0 to $80.0 Billion and Ad
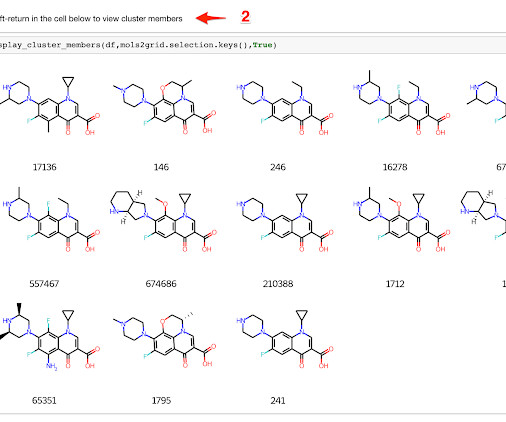
Practical Cheminformatics
JULY 27, 2021
In Cheminformatics, we frequently run into cases where we want to look at leader/follower relationships between chemical structures. For instance, if we've clustered a set of molecules, we might want to start by looking at a table with one example structure for each cluster. We'd then like to be able to select one or more "interesting" clusters and drill down to the cluster members.

Drug Discovery Today
JULY 7, 2021
Cambridge Cognition spin-out targets schizophrenia and post-operative cognitive dysfunction

Eye on FDA
JULY 14, 2021
Upfront Author’s Note: This is the first posting in a while as I have been working to migrate the subscription service to a new provider. As such, some subscribers were lost in the process. Anyone who signed up for a subscription prior to 2019, would need to sign up again. Apologies for the inconvenience and thanks for your patience. Years ago, a single regulatory action letter issued by FDA’s Office of Prescription Drug Promotion (OPDP) would not have merited a blog posting in and o

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
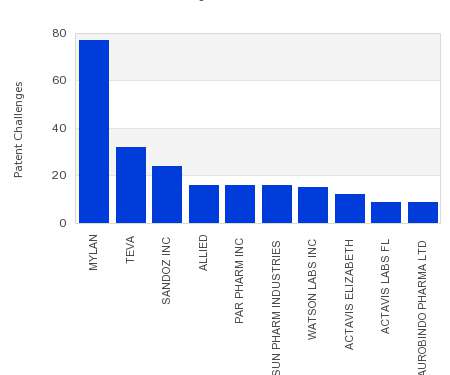
Drug Patent Watch
JULY 26, 2021
This chart shows the generic pharmaceutical companies that had the most successful drug patent challenges from 2016 to 2021. Companies that successfully challenge patents on branded drugs are granted six…. The post Most prolific drug patent challengers appeared first on DrugPatentWatch - Make Better Decisions.

Drug Channels
JULY 27, 2021
Let’s cut through the summer haze with our refreshing breeze of articles and insights. In this issue: The top 10 stingiest hospitals Medicare Part B's savings from biosimilars What happened to hepatitis C drug costs and rebates? Costco beats Medicare Part D Plus, math madness with Duke’s Fuqua business school. P.S. Please join the more than 12,000 consumers of my daily commentary and links to neat stuff at @DrugChannels on Twitter.
The Pharma Data
JULY 29, 2021
Loxo Oncology at Lilly, a research and development group of Eli Lilly and Company (NYSE: LLY), and Kumquat Biosciences today announced an exclusive collaboration focused on the discovery, development and commercialization of potential novel small molecules that stimulate tumor-specific immune responses. Through the multi-year collaboration, Kumquat will utilize its small molecule immuno-oncology (IO) platform to discover novel clinical candidates and Lilly has the option to select a certain numb

The ChEMBL-og
JULY 26, 2021
As mentioned in the announcement post of ChEMBL 29 , a new Drug Warnings Browser has been created. This is an updated version of the entity browsers in ChEMBL ( Compounds , Targets , Activities , etc). It contains new features that will be tried out with the Drug Warnings and will be applied to the other entities gradually. The new features of the Drug Warnings Browser are described below.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.
Pharmaceutical Development Group
JULY 22, 2021
Introduction Food and Drug Administration is a government entity in the United States whose objective is to protect the public by verifying that nutritional supplements are safe to use and accurately labelled. Businesses can even use the FDA Pre-submission to get feedback on possible and planned medical advice, biologics and medication submission. It’s a fantastic service to use, but we have noticed that it’s often overlooked.
Nvidia Developer: Drug Discovery
JULY 21, 2021
Solving a mystery that stumped scientists for decades, last November a group of computational biologists from Alphabet’s DeepMind used AI to predict a. Solving a mystery that stumped scientists for decades, last November a group of computational biologists from Alphabet’s DeepMind used AI to predict a protein’s structure from its amino acid sequence.
Drug Patent Watch
JULY 25, 2021
This chart shows the pharmaceutical companies with the most ointment dosed drugs. For a different perspective, see the most popular dosage types. The companies with the most ointment dosed drugs…. The post Which pharmaceutical companies have the most ointment dosed drugs? appeared first on DrugPatentWatch - Make Better Decisions.

ProRelix Research
JULY 21, 2021
Emergency Use Authorisation Approval Process of US FDA and Treatments Approved for COVID – 19 in the USA The COVID-19 pandemic has transformed the worldwide regulator performing on approval of […]. The post COVID-19 Emergency Use Authorisation Approval Process of US FDA appeared first on ProRelix Research.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

The Pharma Data
JULY 29, 2021
Baricitinib now authorized for emergency use as monotherapy. Eli Lilly and Company (NYSE: LLY) and Incyte (NASDAQ:INCY) announced today the U.S. Food and Drug Administration (FDA) has broadened the Emergency Use Authorization (EUA) for baricitinib to allow for treatment with or without remdesivir, whereas the EUA was previously restricted to use only in combination with remdesivir.
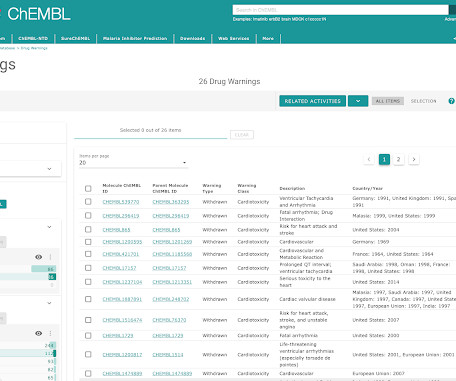
The ChEMBL-og
JULY 23, 2021
We are pleased to announce the release of ChEMBL 29. This version of the database, prepared on 01/07/2021 contains: 2,703,543 compound records 2,105,464 compounds (of which 2,084,724 have mol files) 18,635,916 activities 1,383,553 assays 14,554 targets 81,544 documents Data can be downloaded from the ChEMBL FTP site: [link]. Please see ChEMBL_29 release notes for full details of all changes in this release: [link] New Deposited Datasets EUbOPEN Chemogenomic Library (src_id = 55, ChEMBL Document

Dark Matter Blog
JULY 19, 2021
Back in 2015, I felt compelled to set out in a new direction in drug discovery. I was in my third year as VP of Chemistry for Celgene. This was a great gig – good people, good science. However, it required a level of travel that began to seem excessive. My kids were old enough to know I was missing and young enough to care. And I live in the “Hollywood” of biotech, the Boston/Cambridge area – there seems to be an exciting new biotech start-up in this neighborhood every six weeks!

Drug Channels
JULY 16, 2021
Today’s guest post comes from MMIT's Dinesh Kabaleeswaran, Director of Advisory Services, and John Griggs, Senior Solution Consultant. Dinesh and John discuss the expanding role and importance of integrated delivery networks (IDNs) in specialty medication administration and reimbursement. Dinesh and John also highlight MMIT’s Pulse Analytics solution , which allows pharma manufacturers the ability to monitor drug coverage at the brand and IDN level.
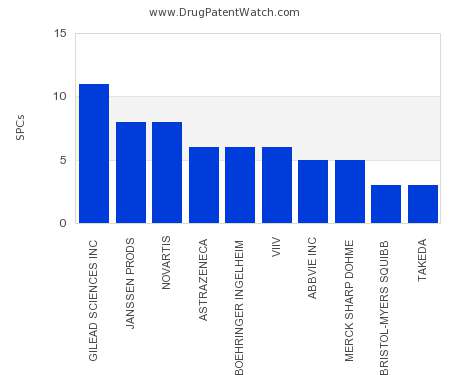
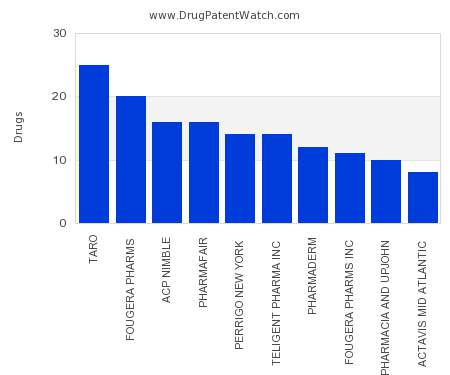
Drug Patent Watch
JULY 24, 2021
This chart shows the pharmaceutical companies with the most supplementary protection certificates (SPCs) in Slovakia. SPCs are used in European Union and select others to encourage pharmaceutical innovation by compensating…. The post Which pharmaceutical companies have the most SPCs in Slovakia? appeared first on DrugPatentWatch - Make Better Decisions.
Let's personalize your content