How are laboratories tackling misinformation?
Drug Discovery Today
SEPTEMBER 16, 2022
Why opening up communication channels can stop the spread of misinformation

Drug Discovery Today
SEPTEMBER 16, 2022
Why opening up communication channels can stop the spread of misinformation

Antidote
SEPTEMBER 30, 2022
Since 1999, October has been recognized as Health Literacy Month. The goal, as defined by the Institute for Healthcare Advancement , is to raise awareness about the importance of understandable health information — but what is health literacy and why is it important?
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
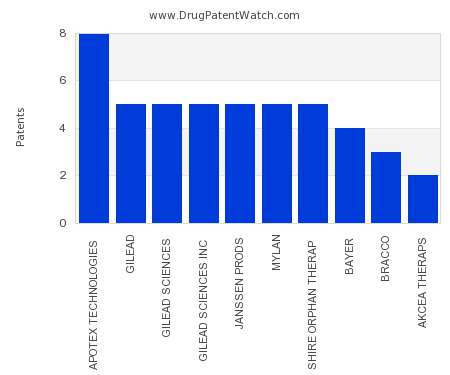
Drug Patent Watch
SEPTEMBER 15, 2022
This chart shows the pharmaceutical companies with the most patents in Ireland. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is…. The post Which pharmaceutical companies have the most drug patents in Ireland? appeared first on DrugPatentWatch - Make Better Decisions.
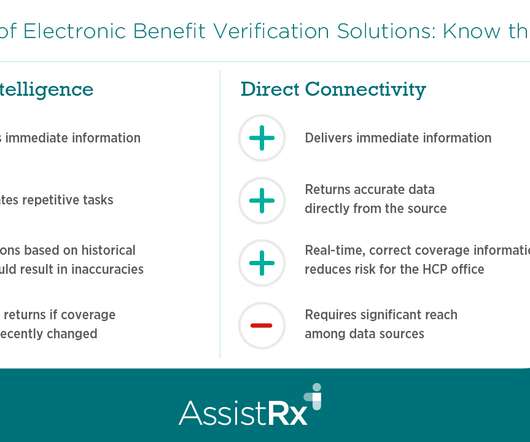
Drug Channels
SEPTEMBER 16, 2022
Today’s guest post comes from Edward Hensley, Chief Commercial Officer at AssistRx. Edward compares the pros and cons of electronic benefit verification using artificial intelligence, direct connectivity, or data connectivity. He also describes the circumstances where each solution provides the greatest value. To learn more, download AssistRx’s free eBook: Specialty Drug Patient Support Programs: 2022 Progress Report.

Speaker: Simran Kaur, Co-founder & CEO at Tattva.Health
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

Eye on FDA
SEPTEMBER 28, 2022
FDA published notice recently that the Office of Prescription Drug Promotion (OPDP) was proposing some new research related to the promotion of medicines by pharmaceutical companies. The research was new (kind of) but the direction was more of the same old focus – direct-to-consumer advertising (DTC). First, just a note on why the research is of importance at all.
Drug Discovery Digest brings together the best content for drug research and development professionals from the widest variety of industry thought leaders.

Drug Discovery Today
SEPTEMBER 9, 2022
On the 26th-27th of October, Vilnius is hosting the European Forum for Industrial Biotechnology & the Bioeconomy 2022 (EFIB'22). The forum will bring together business leaders, entrepreneurs, scientists, and investors as one of Europe's most meaningful events for biotechnology. We caught up with the Executive Director of the Lithuanian Biotechnology Association, Agne Vaitkeviciene, who told us about the importance of the forum and the potential of the Lithuanian life sciences sector.

Antidote
SEPTEMBER 5, 2022
Because Hunter syndrome is a rare, inherited disorder that mostly impacts children, it can lead to many questions from the parents and caregivers of those that are affected.
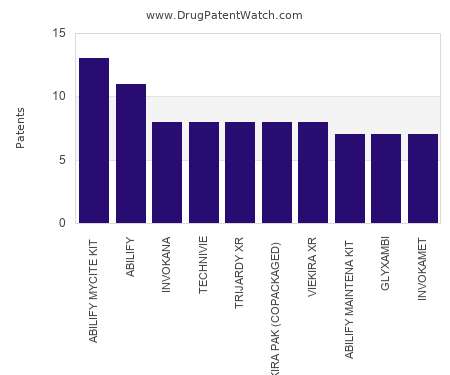
Drug Patent Watch
SEPTEMBER 27, 2022
This chart shows the drugs with the most patents in Ukraine. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is desired.…. The post Which pharmaceutical drugs have the most drug patents in Ukraine? appeared first on DrugPatentWatch - Make Better Decisions.

Drug Channels
SEPTEMBER 7, 2022
As regular readers know, the biosimilar boom for provider-administered drugs has been accelerating. Prices are dropping while adoption accelerates. In many therapeutic areas, biosimilars’ market share is approaching 80%. In the video below, I review these developments and discuss lessons from the launch of Semglee, the first interchangeable insulin.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven
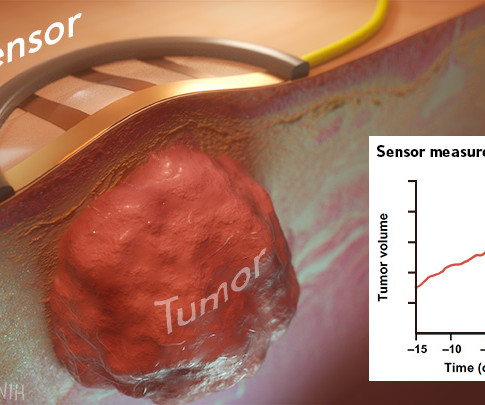
NIH Director's Blog: Drug Development
SEPTEMBER 27, 2022
Wearable electronic sensors hold tremendous promise for improving human health and wellness. That promise already runs the gamut from real-time monitoring of blood pressure and abnormal heart rhythms to measuring alcohol consumption and even administering vaccines. Now a new study published in the journal Science Advances [1] demonstrates the promise of wearables also extends to the laboratory.

Advarra
SEPTEMBER 29, 2022
Informed consent is one of the central protections the regulations provide to research subjects. This tip sheet outlines the regulatory requirements for research informed consent forms (ICFs). The regulatory requirements for informed consent will vary depending upon which regulations apply to the conduct of a particular study. Note: Individual institutional review boards (IRBs) may have their own specific policies regarding how ICFs should be formatted, how to address certain regulatory criteri

Drug Discovery Today
SEPTEMBER 8, 2022
Collaboration expands Lonza’s end-to-end offering for mRNA manufacturing with additional, differentiated source of DNA raw material, Touchlight’s doggybone DNA (dbDNATM). Touchlight provides synthetic DNA through a cell-free enzymatic process, with advantages of speed, quality, capacity, and scalability.

Antidote
SEPTEMBER 12, 2022
There are many factors one should consider before deciding to enroll in a clinical trial. Though every clinical trial is designed to help researchers advance their knowledge about a specific condition, the details regarding a study’s duration, risks, benefits, and required time commitment can vary. When determining how to participate in clinical trials, these elements are all good things to discuss with the research team to determine if a study is a good fit.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
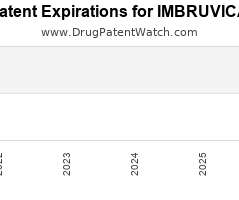
Drug Patent Watch
SEPTEMBER 24, 2022
Annual Drug Patent Expirations for IMBRUVICA Imbruvica is a drug marketed by Pharmacyclics Inc and is included in two NDAs. It is available from one supplier. There are forty patents…. The post New patent for Pharmacyclics Inc drug IMBRUVICA appeared first on DrugPatentWatch - Make Better Decisions.

Drug Channels
SEPTEMBER 9, 2022
Today’s guest post comes from Kristina Crockett, Vice President of Product Management at CoverMyMeds. Kristina discusses the affordability challenges that patients face in our complex and confusing healthcare system. She then explains how data and technology can help payers, manufacturers, and providers improve medication access. To learn more about CoverMyMeds’ comprehensive affordability solutions, download Overcoming Medication Affordability Challenges with Patient-Centered Solutions.

National Institute on Drug Abuse: Nora's Blog
SEPTEMBER 27, 2022
Supporting Needed Research on Recovery mfleming Tue, 09/27/2022 - 13:33 Nora's Blog September 28, 2022 National Recovery Month (Recovery Month), which started in 1989, is a national observance held every September to promote and support new evidence-based treatment and recovery practices, the nation’s strong and proud recovery community, and the dedication of service providers and communities who make recovery in all its forms possible.

Advarra
SEPTEMBER 23, 2022
The Food and Drug Administration (FDA) is the federal entity in the U.S. charged with (among other things) “ensur[ing] that safe and effective drugs are available to improve the health of the people in the United States.” . Before the FDA permits a pharmaceutical drug product to be lawfully marketed, sponsors are required to submit information about the product’s safety and efficacy so the FDA can determine : .

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

Drug Discovery Today
SEPTEMBER 8, 2022
Salipro Biotech strengthens its IP portfolio protecting its innovative, proprietary technology to enable the development of therapeutics against challenging drug targets, including GPCR’s, SLCs and Ion Channels.

Antidote
SEPTEMBER 21, 2022
September 21 is World Alzheimer’s Day , a day of recognition aimed at increasing awareness of and understanding the issues faced by people living with Alzheimer’s. Though there are many conditions that can cause memory loss , Alzheimer’s is one of the most common — it is estimated that 5.4 million Americans are living with the condition , most of whom are over the age of 65.
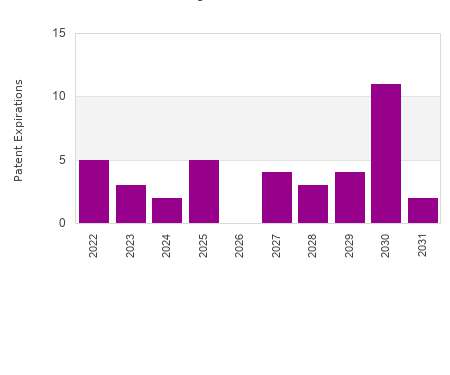
Drug Patent Watch
SEPTEMBER 22, 2022
This chart shows the patent expirations for film dosed drugs over the next decade. The term of drug patents varies. The basic term for a patent is 20 years from…. The post Film dosed drug patent expirations by year appeared first on DrugPatentWatch - Make Better Decisions.

Drug Channels
SEPTEMBER 20, 2022
On October 11, 2022, Drug Channels Institute will release The 2022–23 Economic Report on Pharmaceutical Wholesalers and Specialty Distributors. This report—our thirteenth edition— remains the most comprehensive, fact-based tool for understanding and analyzing the large and growing U.S. pharmaceutical distribution industry. We are providing you with the opportunity to preorder this thoroughly updated and revised 2022-23 edition at special discounted prices.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic
Crown Bioscience
SEPTEMBER 22, 2022
Modern high-content imaging (HCI) and analysis platforms provide simultaneous multi-parameter visualization and quantification of thousands of cells including 3D in vitro models. This highly comprehensive information provides deep insights into the mechanisms of action, toxicity, and synergistic and off-target effects of single and combinations of compounds.
Olympian Clinical Research
SEPTEMBER 22, 2022
It is estimated that about 14% of the population will experience onychomycosis, more commonly known as toenail fungus, at some point in their lives. Toenail fungus may affect people of all ages, but it is more common in adults over 60. Furthermore, it can quickly spread to other toenails if left untreated. This all-inclusive guide will teach you everything you need to know about toenail fungus, from identifying the symptoms to exploring treatment options.

ProRelix Research
SEPTEMBER 21, 2022
According to the World Health Organization (WHO), cancer is the leading cause of death worldwide, with a death rate of one in six in 2020 (1). Aside from the high […]. The post Cancer Clinical Trials: USA Scenario and Study Designs appeared first on ProRelix Research.

Antidote
SEPTEMBER 7, 2022
Strong clinical trial recruitment strategies start from a place of patient centricity, incorporating a variety of outreach methods designed to educate and engage potential participants. Creating a structured clinical trial patient recruitment plan before outreach begins is a smart way to ensure that the process is organized, efficient, and keeps the patient experience as the top priority.
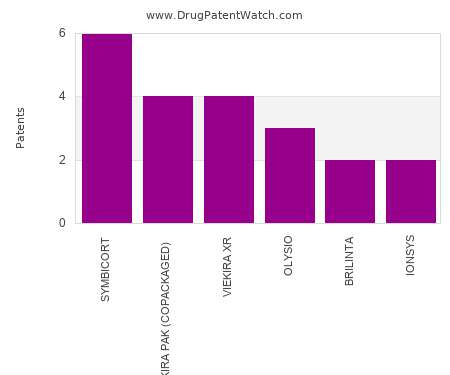
Drug Patent Watch
SEPTEMBER 21, 2022
This chart shows the drugs with the most patents in Sweden. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is desired.…. The post Which pharmaceutical drugs have the most drug patents in Sweden? appeared first on DrugPatentWatch - Make Better Decisions.
Let's personalize your content