Molecular Health and collaborators prove new analytical approach that leverages real-world data to anticipate molecular causation of adverse drug reactions
Drug Discovery Today
MARCH 10, 2022
Heidelberg, 10 March 2022 Molecular Health and collaborators at the University of Florida College of Pharmacy, Takeda Oncology, and thinkQ² published a triad of articles that describe and prove the concept of a novel systems approach to elucidate molecular mechanisms of drug toxicities using real-world data (RWD).






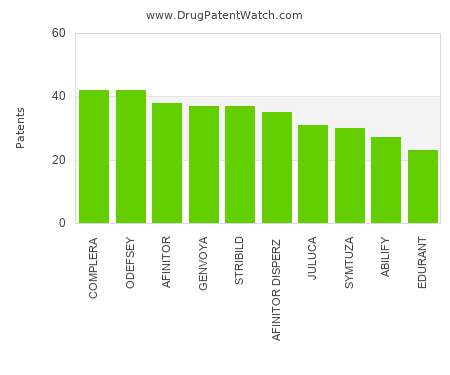













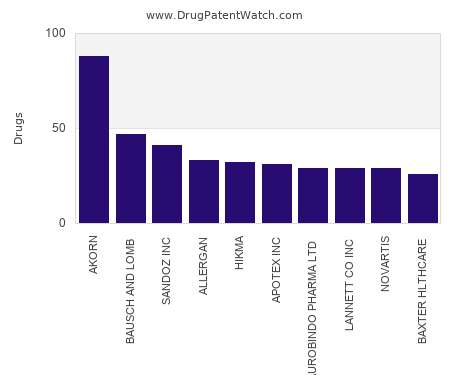












Let's personalize your content