The Current issue of “The view from here” is concerned with Medicinal Chemistry
Drug Discovery Today
AUGUST 15, 2022
The topic of this month’s newsletter from Drug Discovery Today is “Medicinal Chemistry”.

Drug Discovery Today
AUGUST 15, 2022
The topic of this month’s newsletter from Drug Discovery Today is “Medicinal Chemistry”.
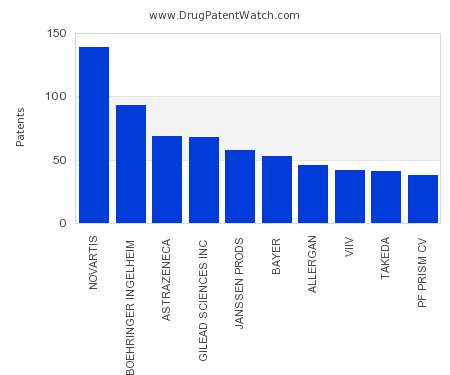
Drug Patent Watch
AUGUST 18, 2022
This chart shows the pharmaceutical companies with the most patents in Norway. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is…. The post Which pharmaceutical companies have the most drug patents in Norway? appeared first on DrugPatentWatch - Make Better Decisions.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Antidote
AUGUST 19, 2022
Celiac disease, an autoimmune disorder that causes a reaction to gluten, is a condition that impacts about 1 in 141 people in the United States. For those with celiac disease, ingesting gluten creates toxins that destroy the villi of the small intestine, which makes it difficult for the body to absorb nutrients from food.

Drug Channels
AUGUST 19, 2022
Today’s guest post comes from H. John Beardsley, Senior Vice President of Corporate Strategy at CoverMyMeds. John discusses how a patient-centric technology solution can increase access and affordability for patients. He goes on to describe how human contact and support helps patients understand their disease and their treatment plan, thus increasing adherence.

Speaker: Simran Kaur, Co-founder & CEO at Tattva.Health
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

Drug Discovery Today
AUGUST 18, 2022
Reading, 12 August 2022. Sanofi (EURONEXT: SAN and NASDAQ: SNY) and the National Institute for Health and Care Research (NIHR) have today announced that the first patient has been enrolled in the Hospitalised RSV Monoclonal Antibody Prevention (HARMONIE) study. The large European interventional clinical study is investigating protection against Respiratory syncytial virus (RSV) in infants.
Drug Discovery Digest brings together the best content for drug research and development professionals from the widest variety of industry thought leaders.

Antidote
AUGUST 17, 2022
Clinical research studies around the United States are in need of participants to learn more about various conditions. For those interested in learning how to take part in research, finding clinical trials that might be a fit can be simple — by answering a few questions about your location and medical history, you can see all the active trials you may be eligible for.
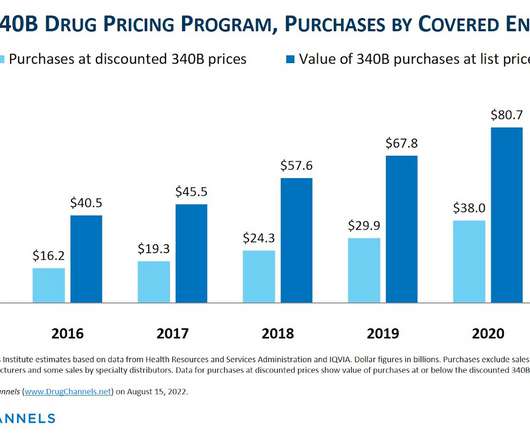
Drug Channels
AUGUST 15, 2022
Here’s a summer surprise for fans of the 340B Drug Pricing Program: Drug Channels has just obtained the 2021 figures from the Health Resources and Services Administration (HRSA)! Even better, my Freedom of Information Act (FOIA) request was able to pry out detailed purchases by covered entity type. The data tell a familiar story. For 2021, discounted purchases under the 340B program reached a record $43.9 billion —an astonishing $5.9 billion (+15.6%) higher than its 2020 counterpart.

Eye on FDA
AUGUST 17, 2022
Periodically I write a posting to look back at what FDA is talking about to get some perspective. Each individual press release tells us something, but looking back at the aggregate can also provide insights. Not long ago, a retrospective look that compared the ratio and trend of COVID versus non-COVID related news out of FDA suggested that not only was COVID becoming less of a topic for the agency, but also presented the possibility that there was increasing room for other topics.
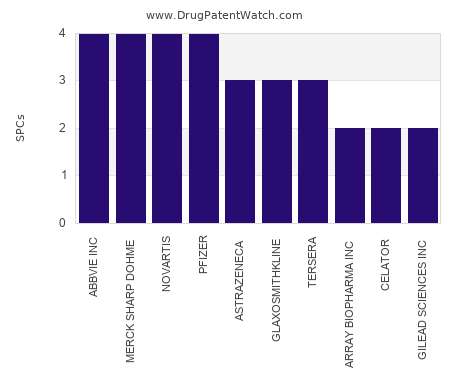
Drug Patent Watch
AUGUST 19, 2022
This chart shows the pharmaceutical companies with the most supplementary protection certificates (SPCs) in Spain. SPCs are used in European Union and select others to encourage pharmaceutical innovation by compensating…. The post Which pharmaceutical companies have the most SPCs in Spain? appeared first on DrugPatentWatch - Make Better Decisions.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven
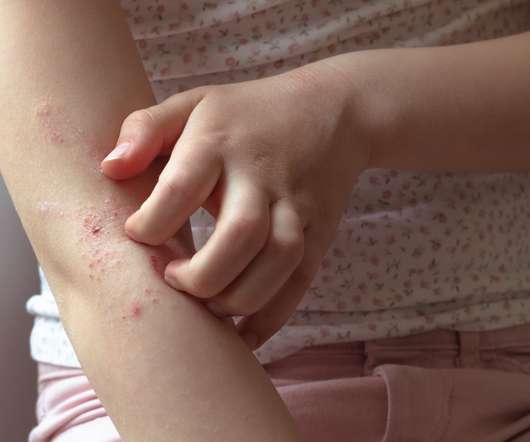
Olympian Clinical Research
AUGUST 19, 2022
Psoriasis and eczema are both chronic conditions that can affect children and adults alike. While these conditions may be similar, there are some distinguishing characteristics. Knowing the difference between pediatric psoriasis and eczema may help you get the correct diagnosis for your child and help them find relief. While these conditions cannot be cured, there are treatments available that may lessen the symptoms.

Crown Bioscience
AUGUST 18, 2022
Are you confident that your cell lines and biological models are really what you think they are? Do you know that common PCR-based cell line authentication services may not be able to detect contamination of up to 20% of your cell line?

Advarra
AUGUST 18, 2022
In the last few years, the clinical research industry has seen a bigger push for electronic informed consent (eConsent) than ever before. Although it’s becoming more widespread, there are currently no definitions for eConsent in the Human Subjects Protection– it’s a concept only described in guidance. eConsent typically refers to the use of electronic systems and processes to: Convey information related to the study, and/or.
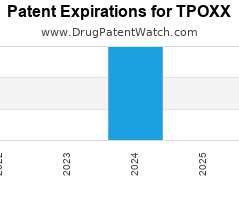
Drug Patent Watch
AUGUST 19, 2022
Annual Drug Patent Expirations for TPOXX Tpoxx is a drug marketed by Siga Technologies and is included in two NDAs. It is available from one supplier. There are nine patents…. The post New patent for Siga Technologies drug TPOXX appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

The ChEMBL-og
AUGUST 16, 2022
We are pleased to announce the release of ChEMBL 31! This version of the database, prepared on 12/07/2022 contains: 2,967,627 compound records 2,331,700 compounds (of which 2,304,875 have mol files) 19,780,369 activities 1,498,681 assays 15,072 targets 85,431 documents New Data Sources MMV Malaria HGL Fraunhofer HDAC6 Updated Data Sources Scientific Literature Patent Bioactivity Data Donated Chemical Probes - SGC Frankfurt EUbOpen Chemogenomic Library Newly Deposited Datasets CHEMBL4888484 - Rep

Antidote
AUGUST 15, 2022
Gaining insight into medical conditions is what clinical trials are all about, but the data acquired is inevitably specific to the study participants — and in many cases, this means that racial and ethnic minorities are underrepresented in the results. Conducting medical studies is vital to discovering new therapies for conditions, but to do this effectively, researchers must ensure that their trial participants accurately reflect the patient population.

Advarra
AUGUST 16, 2022
A rare, or orphan, disease by definition affects a small percentage of the population — fewer than 200,000 people in the U.S. But the numbers add up, and taken together, rare diseases impact an estimated 30 million Americans. Orphan drugs have historically faced a number of barriers, such as limited research and development (R&D) investment due to an expected lack of profitability as well as challenges in clinical trial design and recruitment.
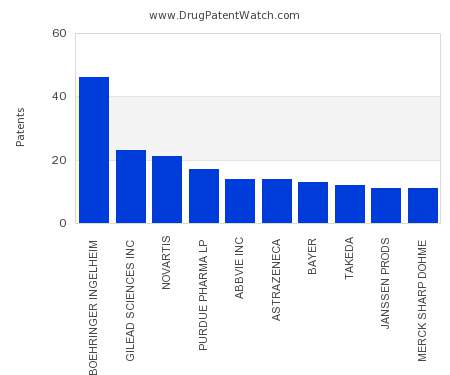
Drug Patent Watch
AUGUST 17, 2022
This chart shows the pharmaceutical companies with the most patents in Serbia. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is…. The post Which pharmaceutical companies have the most drug patents in Serbia? appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

Translation
AUGUST 18, 2022
Social media platforms are a powerful tool to communicate with an audience, advertise a new business or connect with friends. While platforms like LinkedIn and ResearchGate are geared primarily toward the workplace, Twitter also acts as a scientist and startup hotspot. In recent years, researchers and tech startups have taken to Twitter in an attempt to make science more accessible to the general public.

Pharma Manufacturing
AUGUST 16, 2022
Two key facility considerations that will impact pharma’s business case

Drug & Device Law
AUGUST 19, 2022
Earlier this week , we spoke of the impending birth of our soon-to-be standard poodle puppy. We are delighted to report that the puppies are being born as we type this! Eight are expected (e-mail us and we will send you a cool x-ray that shows all eight in utero – count the spines and skulls!), and one has arrived. Dad is white and Mom is silver, and we are told that the litter will likely be a mix of blue (a dark steel grey) and white puppies.

Drug Patent Watch
AUGUST 17, 2022
Annual Drug Patent Expirations for OPZELURA Opzelura is a drug marketed by Incyte Corp and is included in one NDA. It is available from one supplier. There are eleven patents…. The post New patent for Incyte Corp drug OPZELURA appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

Drug Patent Watch
AUGUST 17, 2022
Annual Drug Patent Expirations for AFINITOR+DISPERZ Afinitor Disperz is a drug marketed by Novartis Pharm and is included in one NDA. It is available from one supplier. There are two…. The post New patent expiration for Novartis Pharm drug AFINITOR DISPERZ appeared first on DrugPatentWatch - Make Better Decisions.
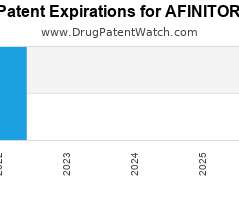
Drug Patent Watch
AUGUST 17, 2022
Annual Drug Patent Expirations for AFINITOR Afinitor is a drug marketed by Novartis and Novartis Pharm and is included in two NDAs. It is available from one supplier. There are…. The post New patent expiration for Novartis drug AFINITOR appeared first on DrugPatentWatch - Make Better Decisions.
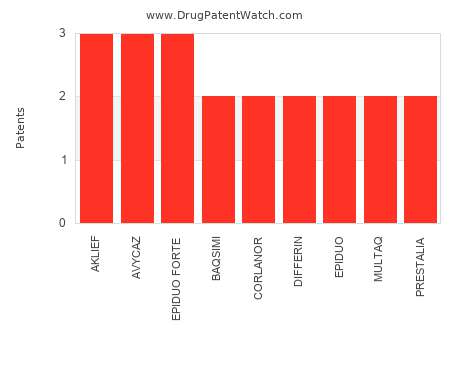
Drug Patent Watch
AUGUST 16, 2022
This chart shows the drugs with the most patents in France. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is desired.…. The post Which pharmaceutical drugs have the most drug patents in France? appeared first on DrugPatentWatch - Make Better Decisions.

Drug Patent Watch
AUGUST 16, 2022
Annual Drug Patent Expirations for GALAFOLD Galafold is a drug marketed by Amicus Therap Us and is included in one NDA. There are forty-one patents protecting this drug. This drug…. The post New patent for Amicus Therap drug GALAFOLD appeared first on DrugPatentWatch - Make Better Decisions.
Drug Patent Watch
AUGUST 16, 2022
AFINITOR (everolimus) Novartis Patent: 8,778,962 Expiration: Aug 18, 2022 See More … For more information on how DrugPatentWatch can help with your pharmaceutical business intelligence needs, contact admin@DrugPatentWatch.com or visit…. The post Drug Patent Expirations for the Week of August 14, 2022 appeared first on DrugPatentWatch - Make Better Decisions.
Let's personalize your content