Dispelling myths about memory loss for World Alzheimer's Day
Antidote
SEPTEMBER 21, 2022
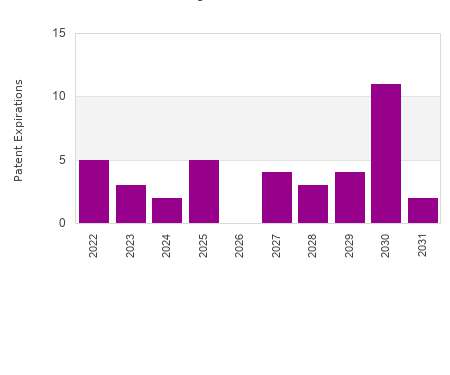
September 21 is World Alzheimer’s Day , a day of recognition aimed at increasing awareness of and understanding the issues faced by people living with Alzheimer’s. Though there are many conditions that can cause memory loss , Alzheimer’s is one of the most common — it is estimated that 5.4 million Americans are living with the condition , most of whom are over the age of 65.
















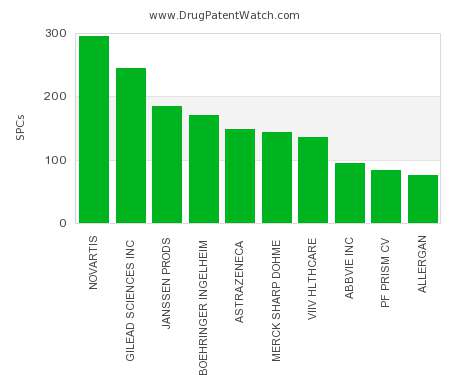
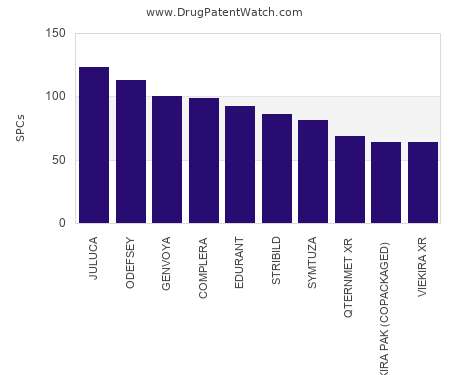
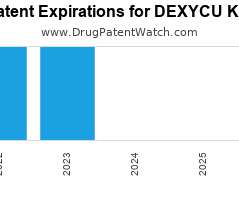
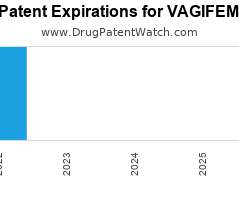







Let's personalize your content