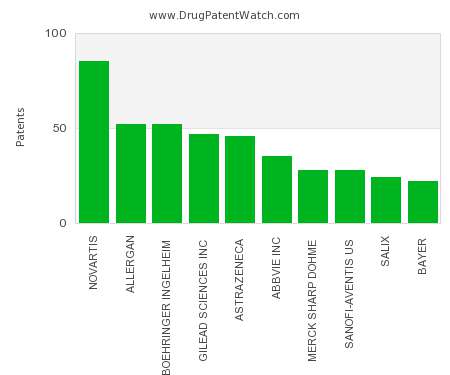
FDA Authorizes Third Dose – Some Implications
Eye on FDA
AUGUST 13, 2021
What does “fully vaccinated” mean today? In the face of the widening spread of the SARS-Cov-2 variant, the rise in breakthrough cases, and data that suggests a slight waning effect over time of vaccines, FDA acted yesterday to protect some of the most vulnerable by amending the Emergency Use Authorization of the mRNA vaccines. People who have had organ transplants and people who are immunocompromised may now get a third dose.



























Let's personalize your content