The Current issue of “The view from here” is concerned with Cardiovascular Drugs
Drug Discovery Today
JULY 8, 2021
The topic of this month’s newsletter from Drug Discovery Today is “Cardiovascular Drugs”.

Drug Discovery Today
JULY 8, 2021
The topic of this month’s newsletter from Drug Discovery Today is “Cardiovascular Drugs”.
Drug Patent Watch
JULY 9, 2021
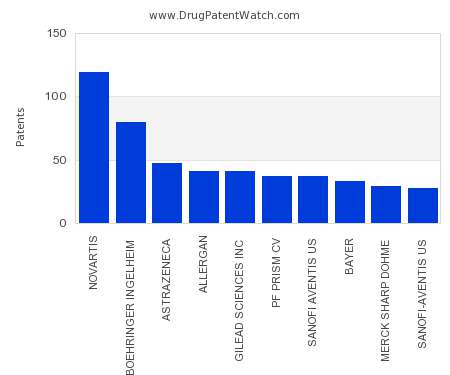
This chart shows the pharmaceutical companies with the most patents in Poland. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is…. The post Which pharmaceutical companies have the most drug patents in Poland? appeared first on DrugPatentWatch - Make Better Decisions.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

The Pharma Data
JULY 8, 2021
Amgen (NASDAQ:AMGN) announced that the U.S. Food and Drug Administration (FDA) has accepted a Biologics License Application (BLA) and granted Priority Review for tezepelumab in the treatment of asthma. Tezepelumab is being developed by Amgen in collaboration with AstraZeneca. The FDA grants Priority Review to applications for medicines that offer significant advantages over available options by demonstrating safety or efficacy improvements, preventing serious conditions or enhancing patient comp

Pharmaceutical Development Group
JULY 7, 2021
Introduction FDA (can also be expanded as the Food and Drug Administration) is a bureau that administers the public’s welfare by controlling and surveillance of food products, medications, vaccines, medical devices, etc. There are certain rules and criteria for post marketing safety reporting of FDA combination products. What are combination products?

Speaker: Simran Kaur, Co-founder & CEO at Tattva.Health
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

Drug Discovery Today
JULY 7, 2021
Cambridge Cognition spin-out targets schizophrenia and post-operative cognitive dysfunction
Drug Discovery Digest brings together the best content for drug research and development professionals from the widest variety of industry thought leaders.

The Pharma Data
JULY 8, 2021
Today, access to the world’s largest browsable resource linking rare protein-coding genetic variants to human health and disease was launched through a genetic exome sequence analysis collaboration between AbbVie (NYSE: ABBV), Biogen Inc. (Nasdaq: BIIB) and Pfizer (NYSE: PFE). Managed by the Broad Institute of MIT and Harvard, the browser gives access to results from analyses of whole exome sequencing data from 300,000 UK Biobank research participants.

Olympian Clinical Research
JULY 7, 2021
Social anxiety disorder, sometimes referred to as a social phobia, is a type of anxiety disorder characterized by extreme fear, distress, and anxiety in social settings. It’s important to note that being naturally more shy, reserved, or introverted is not the same thing as having social anxiety. While people with social anxiety may or may not exhibit shyness in social situations, it’s the uncontrollable fear, anxiety, and subsequent avoidance of social situations that differentiates someone with
Crown Bioscience
JULY 5, 2021
Last month we hosted a webinar with Dr. Ludovic Bourré, Senior Director of Scientific Engagement at Crown Bioscience, focused on the next generation of preclinical models advancing bispecific T cell engager (BiTE) development.

Drug Patent Watch
JULY 7, 2021
Annual Drug Patent Expirations for XHANCE Xhance is a drug marketed by Optinose Us Inc and is included in one NDA. There are thirteen patents protecting this drug. XHANCE drug…. The post New patent for Optinose Us drug XHANCE appeared first on DrugPatentWatch - Make Better Decisions.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

The Pharma Data
JULY 8, 2021
Biogen Inc. (Nasdaq:BIIB) today announced it will report second quarter 2021 financial results Thursday, July 22, 2021, before the financial markets open. Following the release of the financials, the Company will host a live webcast with Biogen management at 8:00 a.m. ET. To access the live webcast, please go to the investors section of Biogen’s website at investors.biogen.com.

Practical Cheminformatics
JULY 7, 2021
Another pointer to the FastPages site

Drug Channels
JULY 7, 2021
ICYMI, Rite Aid recently released the financial results for its most recent fiscal quarter, which ended on May 29. Rite Aid’s pharmacy benefit manager (PBM) business, Elixir, continues to flail in an increasingly challenging market. I’ve long been skeptical of Rite Aid’s PBM strategy. As you will see below, last quarter’s results further validated my skepticism.
Drug Patent Watch
JULY 7, 2021
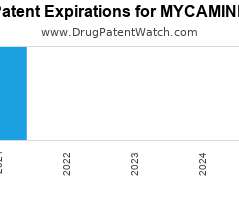
Annual Drug Patent Expirations for MYCAMINE Mycamine is a drug marketed by Astellas and is included in one NDA. It is available from one supplier. There is one patent protecting…. The post New patent expiration for Astellas drug MYCAMINE appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
The Pharma Data
JULY 8, 2021
ADUHELM should be initiated in patients with mild cognitive impairment due to Alzheimer’s disease or mild Alzheimer’s dementia. Biogen (Nasdaq: BIIB) and Eisai Co., Ltd. (Tokyo, Japan) today announced the U.S. Food and Drug Administration (FDA) has approved an updated label for ADUHELM (aducanumab-avwa) injection 100 mg/mL solution. The update includes an addition to the Indications and Usage section of the label (Section 1) to emphasize the disease stages studied in the clinical trials, as seen
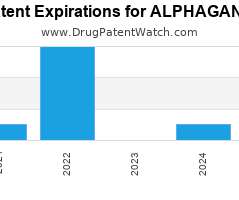
Drug Patent Watch
JULY 9, 2021
Annual Drug Patent Expirations for ALPHAGAN+P Alphagan P is a drug marketed by Allergan and is included in two NDAs. It is available from one supplier. There are eight patents…. The post New patent expiration for Allergan drug ALPHAGAN P appeared first on DrugPatentWatch - Make Better Decisions.
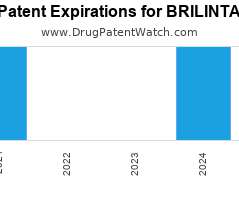
Drug Patent Watch
JULY 8, 2021
Annual Drug Patent Expirations for BRILINTA Brilinta is a drug marketed by Astrazeneca and is included in one NDA. It is available from two suppliers. There are four patents protecting…. The post New patent expiration for Astrazeneca drug BRILINTA appeared first on DrugPatentWatch - Make Better Decisions.
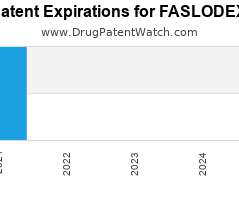
Drug Patent Watch
JULY 8, 2021
Annual Drug Patent Expirations for FASLODEX Faslodex is a drug marketed by Astrazeneca and is included in one NDA. It is available from one supplier. There are four patents protecting…. The post New patent expiration for Astrazeneca drug FASLODEX appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.
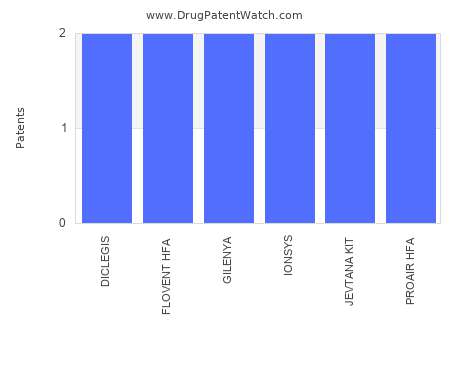
Drug Patent Watch
JULY 8, 2021
This chart shows the drugs with the most patents in Greece. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is desired.…. The post Which pharmaceutical drugs have the most drug patents in Greece? appeared first on DrugPatentWatch - Make Better Decisions.
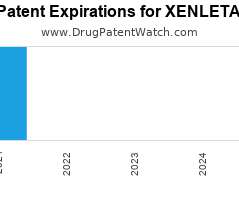
Drug Patent Watch
JULY 8, 2021
Annual Drug Patent Expirations for XENLETA Xenleta is a drug marketed by Nabriva and is included in two NDAs. There are four patents protecting this drug. Drug patent litigation for…. The post New patent expiration for Nabriva drug XENLETA appeared first on DrugPatentWatch - Make Better Decisions.
Drug Patent Watch
JULY 8, 2021
Annual Drug Patent Expirations for MYFEMBREE Myfembree is a drug marketed by Myovant Sciences and is included in one NDA. The generic ingredient in MYFEMBREE is estradiol; norethindrone acetate; relugolix.…. The post New patent for Myovant Sciences drug MYFEMBREE appeared first on DrugPatentWatch - Make Better Decisions.
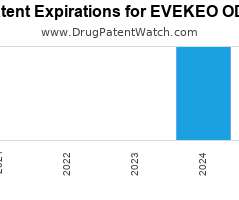
Drug Patent Watch
JULY 8, 2021
Annual Drug Patent Expirations for EVEKEO+ODT Evekeo Odt is a drug marketed by Arbor Pharms Llc and is included in one NDA. It is available from one supplier. There are…. The post New patent for Arbor Pharms drug EVEKEO ODT appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic
The Pharma Data
JULY 8, 2021
People who receive mRNA COVID-19 vaccines are up to 91 percent less likely to develop the disease than those who are unvaccinated, according to a new nationwide study of eight sites, including Salt Lake City. For those few vaccinated people who do still get an infection, or “breakthrough” cases, the study suggests that vaccines reduce the severity of COVID-19 symptoms and shorten its duration.

The Pharma Data
JULY 8, 2021
Johnson & Johnson (NYSE: JNJ) (the Company) announced data that demonstrated its single-shot COVID-19 vaccine generated strong, persistent activity against the rapidly spreading Delta variant and other highly prevalent SARS-CoV-2 viral variants. In addition, the data showed that the durability of the immune response lasted through at least eight months, the length of time evaluated to date.
The Pharma Data
JULY 8, 2021
KEYTRUDA Is Now Approved for the Treatment of Patients With Recurrent or Metastatic or Locally Advanced cSCC That Is Not Curable by Surgery or Radiation. KENILWORTH, N.J.–(BUSINESS WIRE)– Merck (NYSE: MRK), known as MSD outside the United States and Canada, today announced that the U.S. Food and Drug Administration (FDA) has approved an expanded label for KEYTRUDA, Merck’s anti-PD-1 therapy, as monotherapy for the treatment of patients with locally advanced cutaneous squamous cell ca

The Pharma Data
JULY 8, 2021
Tezepelumab is the first and only biologic to consistently and significantly reduce asthma exacerbations in a broad population across Phase II and III clinical trials. AstraZeneca’s Biologics License Application (BLA) for tezepelumab has been accepted and granted Priority Review for the treatment of asthma from the US Food and Drug Administration (FDA).

The Pharma Data
JULY 8, 2021
Takeda Pharmaceutical Company Limited ( TSE:4502/NYSE:TAK ) (“Company”) today announced that it decided to issue new shares (“Issuance of New Shares”) under the Long Term Incentive Plan (“LTIP”) for the Company Group employees overseas, as outlined below. Outline of issuance (1) Payment dateJuly 26, 2021(2) Type and number of shares to be issuedShares of common stock in the Company; numbering 3,874,305 shares(3) Issuance price3,685 yen per share(4) Total value of shar
Let's personalize your content