The Current issue of “The view from here” is concerned with PROTACS
Drug Discovery Today
FEBRUARY 10, 2022
The topic of this month’s newsletter from Drug Discovery Today is “PROTACS”.

Drug Discovery Today
FEBRUARY 10, 2022
The topic of this month’s newsletter from Drug Discovery Today is “PROTACS”.

DrugBaron
FEBRUARY 7, 2022
Finding small molecule drugs is much harder than finding a needle in a haystack – discovering the right arrangement of atoms to bind precisely to a protein target to elicit a particular response is a problem of vast dimensionality. We are most familiar with the numbers involved when dealing with antibodies: a typical antibody library might contain 10 13 different clones – but even that hardly scratches the surface of the 10 80 or so possible CDR sequences.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drug Channels
FEBRUARY 11, 2022
Today’s guest post comes from John Beardsley, Senior Vice President of Corporate Strategy at CoverMyMeds. John discusses some of the findings in CoverMyMeds’ recently published report on medication access. He describes patient-centric, data-driven solutions to help patients access, afford, and adhere to their therapies. To learn more, download CoverMyMeds’ 2022 Medication Access Report.
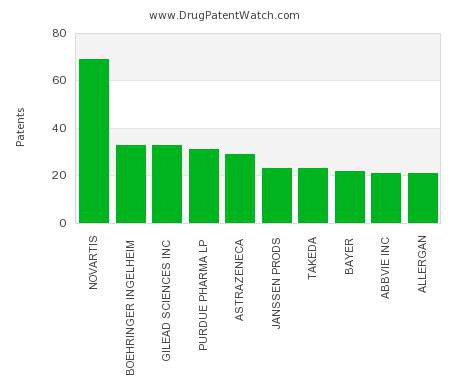
Drug Patent Watch
FEBRUARY 5, 2022
This chart shows the pharmaceutical companies with the most patents in Cyprus. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is…. The post Which pharmaceutical companies have the most drug patents in Cyprus? appeared first on DrugPatentWatch - Make Better Decisions.

Speaker: Simran Kaur, Co-founder & CEO at Tattva.Health
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

ProRelix Research
FEBRUARY 8, 2022
A lot has changed over the past few decades in the way clinical trials are conducted. Advances in data management systems, risk-based monitoring, pharmacovigilance, electronic databases, and application of complex […]. The post Clinical Trial Outsourcing and Management in USA appeared first on ProRelix Research.
Drug Discovery Digest brings together the best content for drug research and development professionals from the widest variety of industry thought leaders.

Drug Channels
FEBRUARY 7, 2022
Drug Pricing Transparency Congress. Hybrid Event March 28-29, 2022 | Philadelphia, PA www.informaconnect.com/drug-pricing-transparency. Don’t miss the Drug Pricing Transparency Congress on March 28-29, 2022! You'll be part of the important discussions on how current and future drug pricing transparency regulations will impact commercialization, reimbursement, pricing and compliance practices.

Sygnature Discovery
FEBRUARY 11, 2022
We’re thrilled to be working with cutting-edge new Dutch biotech company River BioMedics to identify new drugs that directly treat heart failure and cardiovascular disease – the number one cause of death in the world. This exciting partnership combines our novel assay development and hit identification expertise with River BioMedics unique, state-of-the-art technology: advanced human-predictive in vitro 3D heart models, plus their deep knowledge of cardiac biology.
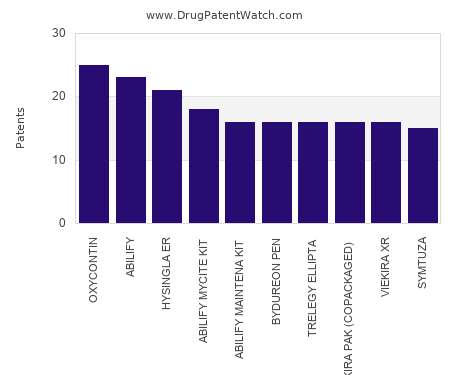
Drug Patent Watch
FEBRUARY 11, 2022
This chart shows the drugs with the most patents in Poland. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is desired.…. The post Which pharmaceutical drugs have the most drug patents in Poland? appeared first on DrugPatentWatch - Make Better Decisions.

Policy Prescription
FEBRUARY 9, 2022
Mark Cuban’s new online pharmacy venture – the Mark Cuban Cost Plus Drug Company (“Cost Plus Drug”) – will not help overcome the biggest obstacle to drug affordability in America, which is drug company monopolies on patented brand name prescription drugs. When only one company makes a drug and can charge as much as the market will bear, which is the case in the United States, the prices are often outrageous, with several treatments exceeding half a million dollars.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Drug Channels
FEBRUARY 8, 2022
More than four years ago, I warned about the emerging trend of copay accumulators and outlined the costly consequences for patients. The latest data reveal that copay accumulator adjustment programs are now in the word list for a growing share of pharmacy benefit designs. What’s more, adoption of copay maximizers now exceeds that of copay accumulators.

Drug & Device Law
FEBRUARY 11, 2022
Today’s case isn’t drug/device, but it’s something our defense-oriented readers should know about. At the tail end of 2021, the Pennsylvania Commonwealth Court laid this rotten egg: Commonwealth v. Monsanto Co. , A.3d , 2021 WL 6139209 (Pa. Cmwlth. Dec. 30, 2021) (“ CvM ”). The Commonwealth Court is a unique Pennsylvania judicial body, mostly devoted to hearing appeals from state governmental bodies, however, it does have original (as opposed to appellate) jurisdiction over certain matters br
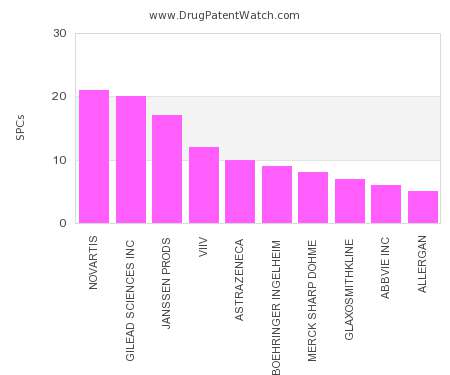
Drug Patent Watch
FEBRUARY 10, 2022
This chart shows the pharmaceutical companies with the most supplementary protection certificates (SPCs) in Germany. SPCs are used in European Union and select others to encourage pharmaceutical innovation by compensating…. The post Which pharmaceutical companies have the most SPCs in Germany? appeared first on DrugPatentWatch - Make Better Decisions.
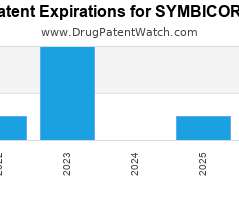
Drug Patent Watch
FEBRUARY 10, 2022
Annual Drug Patent Expirations for SYMBICORT Symbicort is a drug marketed by Astrazeneca and is included in one NDA. It is available from three suppliers. There are ten patents protecting…. The post New patent expiration for Astrazeneca drug SYMBICORT appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
Drug Patent Watch
FEBRUARY 10, 2022
Annual Drug Patent Expirations for BARHEMSYS Barhemsys is a drug marketed by Acacia and is included in one NDA. There are four patents protecting this drug. This drug has thirty-eight…. The post New patent for Acacia drug BARHEMSYS appeared first on DrugPatentWatch - Make Better Decisions.
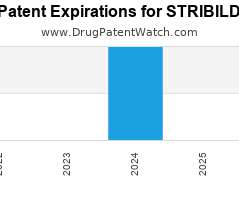
Drug Patent Watch
FEBRUARY 10, 2022
Annual Drug Patent Expirations for STRIBILD Stribild is a drug marketed by Gilead Sciences Inc and is included in one NDA. It is available from one supplier. There are thirteen…. The post New patent for Gilead Sciences drug STRIBILD appeared first on DrugPatentWatch - Make Better Decisions.
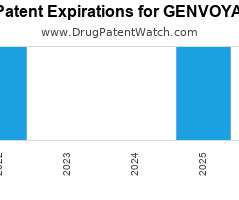
Drug Patent Watch
FEBRUARY 10, 2022
Annual Drug Patent Expirations for GENVOYA Genvoya is a drug marketed by Gilead Sciences Inc and is included in one NDA. It is available from two suppliers. There are thirteen…. The post New patent for Gilead Sciences drug GENVOYA appeared first on DrugPatentWatch - Make Better Decisions.
Drug Patent Watch
FEBRUARY 10, 2022
Annual Drug Patent Expirations for APRETUDE Apretude is a drug marketed by Viiv Hlthcare and is included in one NDA. It is available from one supplier. The generic ingredient in…. The post New patent for Viiv Hlthcare drug APRETUDE appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.
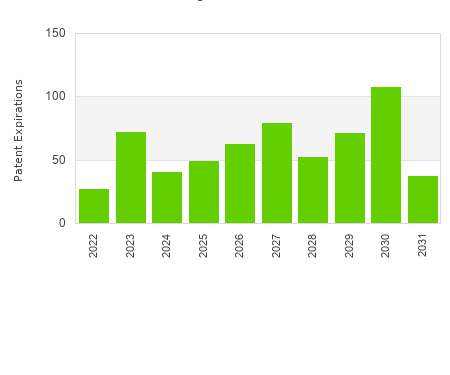
Drug Patent Watch
FEBRUARY 9, 2022
This chart shows the patent expirations for capsule dosed drugs over the next decade. The term of drug patents varies. The basic term for a patent is 20 years from…. The post Capsule dosed drug patent expirations by year appeared first on DrugPatentWatch - Make Better Decisions.
Drug Patent Watch
FEBRUARY 9, 2022
Annual Drug Patent Expirations for OPZELURA Opzelura is a drug marketed by Incyte Corp and is included in one NDA. It is available from one supplier. There are ten patents…. The post New patent for Incyte Corp drug OPZELURA appeared first on DrugPatentWatch - Make Better Decisions.
Drug Patent Watch
FEBRUARY 9, 2022
Annual Drug Patent Expirations for EPANED Epaned is a drug marketed by Azurity and Silvergate Pharms and is included in two NDAs. It is available from one supplier. There are…. The post New patent for Azurity drug EPANED appeared first on DrugPatentWatch - Make Better Decisions.

Drug Patent Watch
FEBRUARY 8, 2022
Annual Drug Patent Expirations for DESCOVY Descovy is a drug marketed by Gilead Sciences Inc and is included in one NDA. It is available from two suppliers. There are six…. The post New patent for Gilead Sciences drug DESCOVY appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

Drug Patent Watch
FEBRUARY 8, 2022
This chart shows the pharmaceutical companies with the most supplementary protection certificates (SPCs) in Estonia. SPCs are used in European Union and select others to encourage pharmaceutical innovation by compensating…. The post Which pharmaceutical companies have the most SPCs in Estonia? appeared first on DrugPatentWatch - Make Better Decisions.

Drug Patent Watch
FEBRUARY 8, 2022
Annual Drug Patent Expirations for CAPLYTA Caplyta is a drug marketed by Intra-cellular and is included in one NDA. There are eleven patents protecting this drug. CAPLYTA drug price trends.…. The post New patent for Intra-cellular drug CAPLYTA appeared first on DrugPatentWatch - Make Better Decisions.
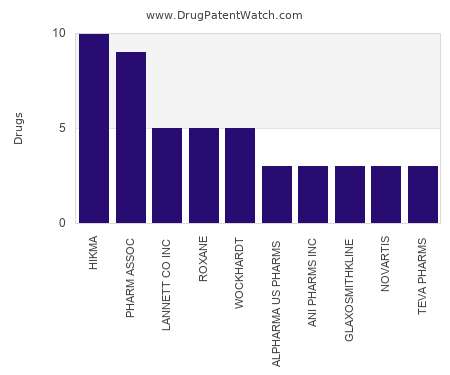
Drug Patent Watch
FEBRUARY 7, 2022
This chart shows the pharmaceutical companies with the most concentrate dosed drugs. For a different perspective, see the most popular dosage types. The companies with the most concentrate dosed drugs…. The post Which pharmaceutical companies have the most concentrate dosed drugs? appeared first on DrugPatentWatch - Make Better Decisions.

Drug Patent Watch
FEBRUARY 7, 2022
Annual Drug Patent Expirations for UKONIQ Ukoniq is a drug marketed by Tg Theraps and is included in one NDA. There are seven patents protecting this drug. This drug has…. The post New patent for Tg Theraps drug UKONIQ appeared first on DrugPatentWatch - Make Better Decisions.
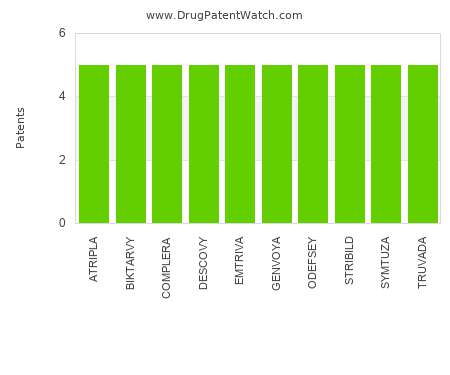
Drug Patent Watch
FEBRUARY 6, 2022
This chart shows the drugs with the most patents in Ireland. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is desired.…. The post Which pharmaceutical drugs have the most drug patents in Ireland? appeared first on DrugPatentWatch - Make Better Decisions.
Let's personalize your content