All eyes on Lab Innovations 2021
Drug Discovery Today
JULY 18, 2021
~ UK’s biggest trade show for the laboratory industry returns for 2021 ~

Drug Discovery Today
JULY 18, 2021
~ UK’s biggest trade show for the laboratory industry returns for 2021 ~
Drug Patent Watch
JULY 22, 2021
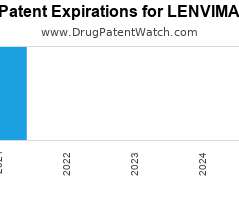
Annual Drug Patent Expirations for LENVIMA Lenvima is a drug marketed by Eisai Inc and is included in one NDA. There are five patents protecting this drug and one Paragraph…. The post New patent for Eisai Inc drug LENVIMA appeared first on DrugPatentWatch - Make Better Decisions.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Eye on FDA
JULY 19, 2021
It has been a regular feature of the blog to give a read on what FDA has been talking about, at least through the form of press releases. It is not always an easy task because the nature of FDA’s practice in this respect has evolved over time. But here is what we see looking at the first 6 months 2021, and comparing it to mid-years past. You may recall from the last posting on this topic at the beginning of the year, during 2020, FDA had a lot to say – a real lot, and that isn’
The ChEMBL-og
JULY 23, 2021
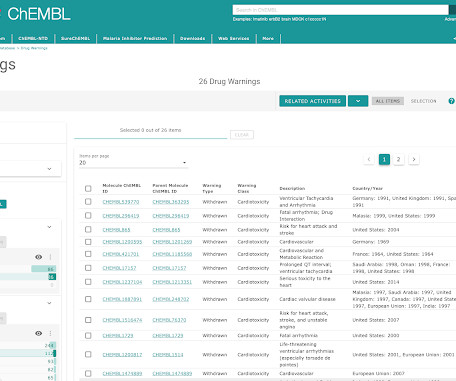
We are pleased to announce the release of ChEMBL 29. This version of the database, prepared on 01/07/2021 contains: 2,703,543 compound records 2,105,464 compounds (of which 2,084,724 have mol files) 18,635,916 activities 1,383,553 assays 14,554 targets 81,544 documents Data can be downloaded from the ChEMBL FTP site: [link]. Please see ChEMBL_29 release notes for full details of all changes in this release: [link] New Deposited Datasets EUbOPEN Chemogenomic Library (src_id = 55, ChEMBL Document

Speaker: Simran Kaur, Co-founder & CEO at Tattva.Health
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

The Pharma Data
JULY 23, 2021
Roche (SIX: RO, ROG; OTCQX: RHHBY) announced that Japan’s Ministry of Health, Labour and Welfare (MHLW) has approved Ronapreve (casirivimab and imdevimab), for the treatment of patients with mild to moderate COVID-19 via intravenous infusion. The antibody combination was granted a Special Approval Pathway under article 14-3 of the Pharmaceuticals and Medical Devices Act. “Ronapreve has been shown to improve survival in high-risk, non-hospitalised COVID-19 patients by reducing the risk of h
Drug Discovery Digest brings together the best content for drug research and development professionals from the widest variety of industry thought leaders.
Pharmaceutical Development Group
JULY 22, 2021
Introduction Food and Drug Administration is a government entity in the United States whose objective is to protect the public by verifying that nutritional supplements are safe to use and accurately labelled. Businesses can even use the FDA Pre-submission to get feedback on possible and planned medical advice, biologics and medication submission. It’s a fantastic service to use, but we have noticed that it’s often overlooked.
Nvidia Developer: Drug Discovery
JULY 21, 2021
Solving a mystery that stumped scientists for decades, last November a group of computational biologists from Alphabet’s DeepMind used AI to predict a. Solving a mystery that stumped scientists for decades, last November a group of computational biologists from Alphabet’s DeepMind used AI to predict a protein’s structure from its amino acid sequence.
The Pharma Data
JULY 23, 2021
Pfizer Inc. (NYSE: PFE) and BioNTech SE (Nasdaq: BNTX) today announced that the U.S. government has purchased an additional 200 million doses of the Pfizer-BioNTech COVID-19 Vaccine. These doses are expected to be delivered from October 2021 through April 2022. This brings the total number of doses to be supplied by the companies to the U.S. government under its existing supply agreement to 500 million.

Drug Patent Watch
JULY 19, 2021
This chart shows the companies which have received the most New Combination exclusivities in the past five years. New Combinations are one of the categories for which the FDA grants…. The post Pharmaceutical companies with the most ‘New Combination’ drugs appeared first on DrugPatentWatch - Make Better Decisions.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

ProRelix Research
JULY 21, 2021
Emergency Use Authorisation Approval Process of US FDA and Treatments Approved for COVID – 19 in the USA The COVID-19 pandemic has transformed the worldwide regulator performing on approval of […]. The post COVID-19 Emergency Use Authorisation Approval Process of US FDA appeared first on ProRelix Research.

Dark Matter Blog
JULY 19, 2021
Back in 2015, I felt compelled to set out in a new direction in drug discovery. I was in my third year as VP of Chemistry for Celgene. This was a great gig – good people, good science. However, it required a level of travel that began to seem excessive. My kids were old enough to know I was missing and young enough to care. And I live in the “Hollywood” of biotech, the Boston/Cambridge area – there seems to be an exciting new biotech start-up in this neighborhood every six weeks!
The Pharma Data
JULY 23, 2021
Real-world data from Canada showed 82% and 87% effectiveness after one dose against hospitalisation or death caused by Beta/Gamma and Delta variants respectively. Results from the Canadian Immunization Research Network (CIRN) with support from Public Health Agency of Canada and the Canadian Institutes of Health Research, published as a pre-print , demonstrated one dose of Vaxzevria was 82% effective against hospitalisation or death caused by the Beta/Gamma variants of the SARS-CoV-2 virus.
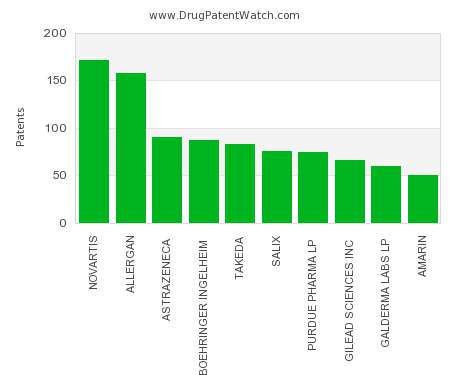
Drug Patent Watch
JULY 23, 2021
A strong patent portfolio is at the core of branded pharmaceutical firms. Accordingly, one way to assess the relative strength of pharmaceutical firms is to compare their patent-counts. This chart…. The post Which pharmaceutical companies have the largest patent portfolios? appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Drug Channels
JULY 20, 2021
The 340B Drug Pricing Program continues to expand far more quickly than the overall pharmaceutical market—and some channels are benefiting more than others. As you will see below, mail and specialty pharmacies’ purchases of products that are eligible for 340B discounts have grown by an incredible 56% per year since 2017. That’s about six times faster than the overall mail and specialty market.

Dark Matter Blog
JULY 19, 2021
Arrakis has gone through many significant transitions since its founding in 2015. We’ve evolved and matured scientifically. We’ve added investors and partners. And, of course, people come and go. Today, we reach a bittersweet transition at Arrakis. We bid our head of research and employee #005, Jim Barsoum, au revoir , as he marches bravely into a well-earned retirement.
The Pharma Data
JULY 23, 2021
– DALVANCE® is the first and only single-dose infusion to treat acute bacterial skin and skin structure infections (ABSSSI) in pediatric patients from birth. AbbVie (NYSE: ABBV) today announced that the U.S. Food and Drug Administration (FDA) approved DALVANCE® (dalbavancin) for the treatment of acute bacterial skin and skin structure infections (ABSSSI) in pediatric patients from birth.
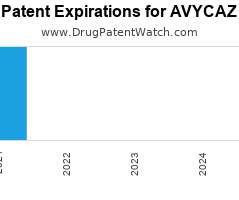
Drug Patent Watch
JULY 23, 2021
Annual Drug Patent Expirations for AVYCAZ Avycaz is a drug marketed by Allergan and is included in one NDA. It is available from one supplier. There are eight patents protecting…. The post New patent expiration for Allergan drug AVYCAZ appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

Drug Channels
JULY 19, 2021
Finance and Accounting for Bioscience Companies – Hybrid Events. September 20-21, Boston MA | September 22, Virtual Experience | September 23-24, Burlingame, CA. www.informaconnect.com/financeaccountingeast. www.informaconnect.com/financeaccountingwest. Tailored to the unique nuisances of bioscience companies, these CPE-accredited annual events bring together the brightest financial and accounting minds from the nation’s leading trailblazers.
The Pharma Data
JULY 23, 2021
In consultation with the U.S. Food and Drug Administration (FDA), Bristol Myers Squibb has made the difficult decision to voluntarily withdraw the indication for Opdivo (nivolumab) as a single agent for patients with hepatocellular carcinoma (HCC) who were previously treated with sorafenib from the U.S. market. We took this action following the Agency’s industry-wide evaluation of accelerated approvals for checkpoint inhibitors that have not met their post-marketing requirements demonstrating co
The Pharma Data
JULY 23, 2021
Biogen Inc. (Nasdaq: BIIB) today announced it will share multiple oral and poster presentations from its Alzheimer’s disease clinical development portfolio at the Alzheimer’s Association International Conference (AAIC), which will be held in Denver, Colorado and online July 26-30, 2021. The company’s contributions to AAIC will include several presentations highlighting data on ADUHELM (aducanumab-avwa) injection 100 mg/mL solution, which was recently granted accelerated approval by the U.S.
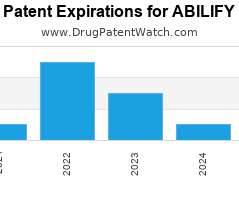
Drug Patent Watch
JULY 23, 2021
Annual Drug Patent Expirations for ABILIFY Abilify is a drug marketed by Otsuka and Otsuka Pharm Co Ltd and is included in six NDAs. It is available from two suppliers.…. The post New patent expiration for Otsuka drug ABILIFY appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic
The Pharma Data
JULY 22, 2021
Some antibiotics appear to be effective against a form of skin cancer known as melanoma. Researchers at KU Leuven, Belgium, examined the effect of these antibiotics on patient-derived tumours in mice. Their findings were published in the Journal of Experimental Medicine. Researchers from KU Leuven may have found a new weapon in the fight against melanoma: antibiotics that target the ‘power plants’ of cancer cells.
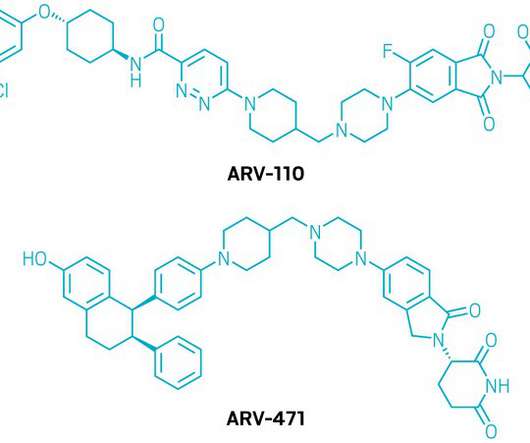
The Pharma Data
JULY 22, 2021
Collaboration combines Arvinas’ investigational estrogen receptor-targeting breast cancer therapy with Pfizer’s deep experience in breast oncology therapeutics – – ARV-471 is currently in Phase 2 development for the treatment of patients with locally advanced or metastatic ER+/HER2- breast cancer – – Arvinas to receive $650 million in an upfront payment, in addition to a potential $1.4 billion in milestone payments; profits and costs to be shared 50/50 worldwide – – Pfizer to complete a $350 mil

The Pharma Data
JULY 22, 2021
The Board of GSK (GSK) today announces that Brian McNamara, the CEO of GSK Consumer Healthcare (a Joint Venture between GSK and Pfizer) has been appointed as CEO Designate of the new, listed Consumer Healthcare company which will result from the proposed demerger of Consumer Healthcare from GSK in 2022. As set out at GSK’s Investor Update on 23 June 2021, subject to approval from shareholders, the separation of Consumer Healthcare will be by way of a demerger in mid-2022 of at least 80% of GSK’s

The Pharma Data
JULY 22, 2021
Immunotherapy and Tyrosine Kinase Inhibitor Combination Approved for the Treatment of Patients With Advanced Endometrial Carcinoma That is Not Microsatellite Instability-High or Mismatch Repair Deficient, Who Have Disease Progression Following Prior Systemic Therapy in Any Setting and Are Not Candidates for Curative Surgery or Radiation. Study Results Demonstrated Statistically Significant Improvements in Overall Survival, Progression-Free Survival and Overall Response Rate, Helping to Address a
The Pharma Data
JULY 22, 2021
Data Include Phase 3 Results Showing that the Primary Endpoint of Spontaneous, Treated Annualized Bleeding Rates During Prophylaxis with Recombinant von Willebrand Factor in Adults with von Willebrand Disease, was Met 12 Abstracts Presented Across Takeda’s Hematology Portfolio and Pipeline Support Takeda’s Commitment to Improving Patient Care Today as well as Transforming Future Care.
Let's personalize your content