The Current issue of “The view from here” is concerned with Therapeutics
Drug Discovery Today
JANUARY 6, 2022
The topic of this month’s newsletter from Drug Discovery Today is “Therapeutics”.

Drug Discovery Today
JANUARY 6, 2022
The topic of this month’s newsletter from Drug Discovery Today is “Therapeutics”.
Drug Patent Watch
JANUARY 2, 2022
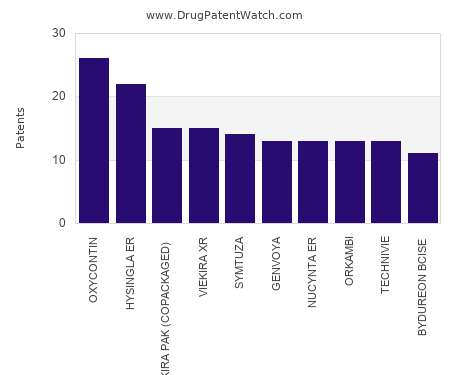
This chart shows the drugs with the most patents in Slovenia. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is desired.…. The post Which pharmaceutical drugs have the most drug patents in Slovenia? appeared first on DrugPatentWatch - Make Better Decisions.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Eye on FDA
JANUARY 5, 2022
FDA has scheduled the first advisory committee meetings of 2022. One meeting of the Oncologic Drugs Advisory Committee is set for February 10 to discuss a new application for the proposed treatment of Non-Small Cell Lung Cancer (NSCLC. And a meeting of the Anesthetic and Analgesic Drug Products Advisory Committee is meeting jointly with the Drug Safety and Risk Management Advisory Committee on February 15 to consider an NDA in the pain category.

Sygnature Discovery
JANUARY 7, 2022
We’ve strengthened our drug discovery offering even further – by licensing industry-leading software for lead optimisation. We’ve deployed the MCPairs platform from MedChemica , which uses anonymised data from major pharmaceuticals to extract rules that can then guide the medicinal chemistry process. Our scientists are now using the tool to help automate directed idea generation.

Speaker: Simran Kaur, Co-founder & CEO at Tattva.Health
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

Drug Discovery Today
JANUARY 6, 2022
Award-winning platform company, Iceni Glycoscience (formerly Iceni Diagnostics) is to broaden the scope of its operations within the field of glycoscience (carbohydrate chemistry and biology) as the role of carbohydrates in human disease becomes increasingly apparent.
Drug Discovery Digest brings together the best content for drug research and development professionals from the widest variety of industry thought leaders.

Pharmaceutical Development Group
JANUARY 7, 2022
Start Up and Generic Pharmaceutical Drug and Biologic Companies have high quality, affordable products and biosimilars that improve the quality of life for their patients. However, this often is associated with austere conditions wherein time and funds are both in short supply. Effective and consistent use and application of Data Standards can reduce costs of Pharmaceutical Drug and Biologic Products and Process Development, Drug Development Services, 505(b) NDA, IND Consulting , NDA Consulting,

PerkinElmer
JANUARY 7, 2022
The pharmaceutical industry is under huge pressure to address the high attrition rates in drug development. With around 90% of candidates failing during clinical development, 1 the process is not only long and risky, but also expensive for those involved. There are many reasons that promising drug candidates are discontinued, including poor pharmacokinetics, lack of clinical efficacy, and toxicity.
Plenge Gen
JANUARY 6, 2022
[ I am an employee of Bristol Myers Squibb. The views expressed here are my own, assuming I am real and not a humanoid. ] In the original Blade Runner (1982), Harrison Ford’s character, Deckard, implements a fictitious Voight-Kampff test to measure bodily functions such as heart rate and pupillary dilation in response to emotionally provocative questions.
Drug Patent Watch
JANUARY 7, 2022
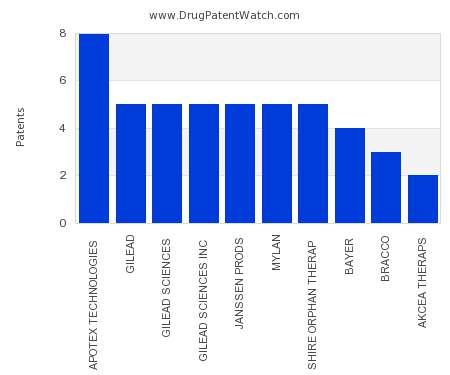
This chart shows the pharmaceutical companies with the most patents in Ireland. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is…. The post Which pharmaceutical companies have the most drug patents in Ireland? appeared first on DrugPatentWatch - Make Better Decisions.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Olympian Clinical Research
JANUARY 5, 2022
When a loved one is diagnosed with Alzheimer’s disease , it can be challenging to know what to do or how to cope. You may first want to understand this degenerative disease so that you can best support your loved one after their Alzheimer’s diagnosis. What is Alzheimer’s Disease? . Alzheimer’s disease is a progressive brain disorder that causes a gradual decline of a person’s mental functions, resulting in memory loss and an inability to think clearly.

Pharmaceutical Development Group
JANUARY 5, 2022
The FDA expects and requires that Sterile Injectable Products adhere to strict FDA standards pertaining to quality, purity, safety, and efficacy. As a result, Patients and Physicians rely upon and trust that the Injectable Products they administer adhere to the same high standards. The cGMP Injectable Product Manufacturing process is generally the primary place where fibers, dust, rubber, shards, metallic flakes, and silicone may enter into Injectable and other Drug Products.

Practical Cheminformatics
JANUARY 3, 2022
There's a lot of useful functionality in the RDKit. My problem is remembering where all of the most useful bits are, and how to use them. In order to make my life, and perhaps yours, a little easier, I put together a Python package called " useful_rdkit_utils ". Some of what's in there is simply a repackaging of existing functionality to make it easier to use (at least for me).
Drug Patent Watch
JANUARY 7, 2022
Annual Drug Patent Expirations for XYOSTED+%28AUTOINJECTOR%29 Xyosted (autoinjector) is a drug marketed by Antares Pharma Inc and is included in one NDA. It is available from one supplier. There are…. The post New patent for Antares Pharma drug XYOSTED (AUTOINJECTOR) appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Drug Channels
JANUARY 4, 2022
Reality has again failed to cooperate with the politically motivated cries of “skyrocketing drug prices” or anecdotes about companies “jacking up prices” (as President Biden recently claimed). Brand-name drug prices continue to decline, while the prices of other healthcare products and services continue to rise. For 2021, brand-name drugs’ net prices dropped for the fourth consecutive year.
Drug Patent Watch
JANUARY 7, 2022
Annual Drug Patent Expirations for ICLUSIG Iclusig is a drug marketed by Takeda Pharms Usa and is included in one NDA. It is available from one supplier. There are three…. The post New patent for Takeda Pharms drug ICLUSIG appeared first on DrugPatentWatch - Make Better Decisions.

Drug Patent Watch
JANUARY 6, 2022
Annual Drug Patent Expirations for EUCRISA Eucrisa is a drug marketed by Anacor Pharms Inc and is included in one NDA. It is available from one supplier. There are four…. The post New patent for Anacor Pharms drug EUCRISA appeared first on DrugPatentWatch - Make Better Decisions.

Drug Patent Watch
JANUARY 6, 2022
This chart shows the drugs with the most patents in Portugal. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is desired.…. The post Which pharmaceutical drugs have the most drug patents in Portugal? appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.
Drug Patent Watch
JANUARY 6, 2022
Annual Drug Patent Expirations for CAPLYTA Caplyta is a drug marketed by Intra-cellular and is included in one NDA. There are nine patents protecting this drug. CAPLYTA drug price trends.…. The post New patent for Intra-cellular drug CAPLYTA appeared first on DrugPatentWatch - Make Better Decisions.
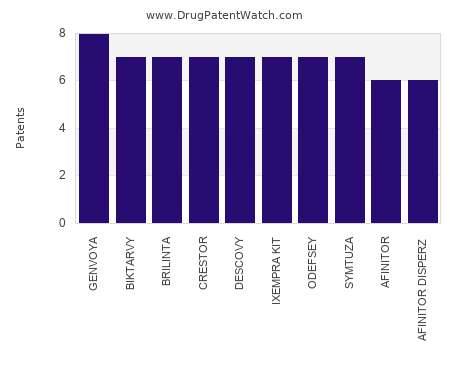
Drug Patent Watch
JANUARY 5, 2022
This chart shows the drugs with the most patents in Czech Republic. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is…. The post Which pharmaceutical drugs have the most drug patents in Czech Republic? appeared first on DrugPatentWatch - Make Better Decisions.
Drug Patent Watch
JANUARY 5, 2022
Annual Drug Patent Expirations for GIAPREZA Giapreza is a drug marketed by La Jolla Pharma and is included in one NDA. It is available from one supplier. There are nine…. The post New patent for La Jolla drug GIAPREZA appeared first on DrugPatentWatch - Make Better Decisions.
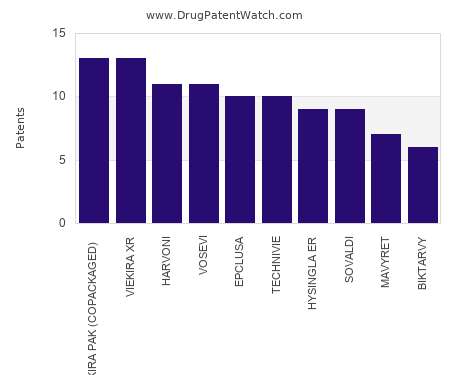
Drug Patent Watch
JANUARY 4, 2022
This chart shows the drugs with the most patents in Chile. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is desired.…. The post Which pharmaceutical drugs have the most drug patents in Chile? appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic
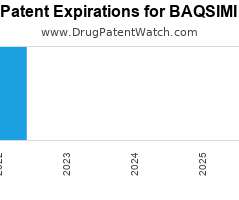
Drug Patent Watch
JANUARY 2, 2022
Annual Drug Patent Expirations for BAQSIMI Baqsimi is a drug marketed by Eli Lilly And Co and is included in one NDA. It is available from one supplier. There are…. The post New patent expiration for Eli Lilly drug BAQSIMI appeared first on DrugPatentWatch - Make Better Decisions.
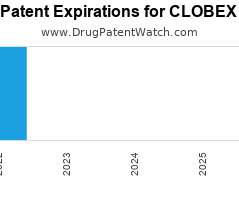
Drug Patent Watch
JANUARY 2, 2022
Annual Drug Patent Expirations for CLOBEX Clobex is a drug marketed by Galderma Labs Lp and Galderma Labs and is included in three NDAs. It is available from four suppliers.…. The post New patent expiration for Galderma Labs drug CLOBEX appeared first on DrugPatentWatch - Make Better Decisions.
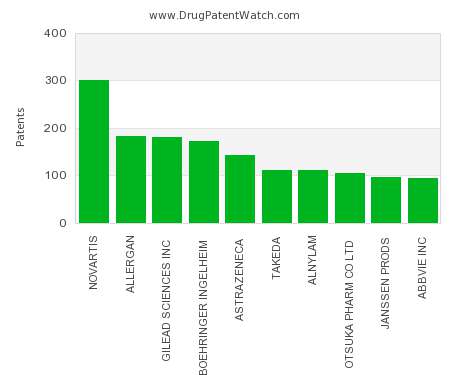
Drug Patent Watch
JANUARY 1, 2022
This chart shows the pharmaceutical companies with the most patents in Japan. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is…. The post Which pharmaceutical companies have the most drug patents in Japan? appeared first on DrugPatentWatch - Make Better Decisions.
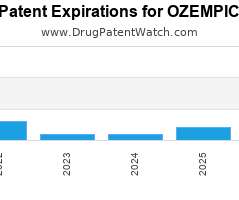
Drug Patent Watch
JANUARY 1, 2022
Annual Drug Patent Expirations for OZEMPIC Ozempic is a drug marketed by Novo and is included in one NDA. It is available from three suppliers. There are twenty-three patents protecting…. The post New patent expiration for NOVO drug OZEMPIC appeared first on DrugPatentWatch - Make Better Decisions.

Drug Patent Watch
JANUARY 2, 2022
OZEMPIC (semaglutide) Novo Patent: 9,486,588 Expiration: Jan 2, 2022 See More … For more information on how DrugPatentWatch can help with your pharmaceutical business intelligence needs, contact admin@DrugPatentWatch.com or visit…. The post Drug Patent Expirations for the Week of January 2, 2022 appeared first on DrugPatentWatch - Make Better Decisions.
Let's personalize your content