The Current issue of “The view from here” is concerned with Epigenetics
Drug Discovery Today
MARCH 30, 2022
The topic of this month’s newsletter from Drug Discovery Today is “Epigenetics”.

Drug Discovery Today
MARCH 30, 2022
The topic of this month’s newsletter from Drug Discovery Today is “Epigenetics”.

Drug Channels
APRIL 1, 2022
Today’s guest post comes from Fauzea Hussain, Vice President of Public Policy at McKesson. Fauzea succinctly describes the complicated and contradictory aspects of the Centers for Medicare & Medicaid Services' Final Rule on Best Price, a.k.a. the Medicaid copay accumulator rule. She then outlines how the rule, if implemented, is likely to hurt patients.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Eye on FDA
MARCH 30, 2022
Tectonic pandemic plates are shifting respecting the COVID-19 pandemic. For weeks now we have been watching caseloads in the United States broadly fall. It feels as if we are indeed shifting gears. There are other moving parts that are in motion as well. It is a fundamental re-shifting of the landscape. And there are consequences. First, government funding supporting access to vaccination and treatment for COVID has run out and so far, is not replenished.
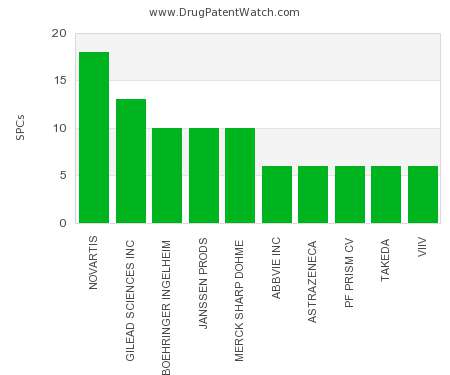
Drug Patent Watch
APRIL 1, 2022
This chart shows the pharmaceutical companies with the most supplementary protection certificates (SPCs) in Ireland. SPCs are used in European Union and select others to encourage pharmaceutical innovation by compensating…. The post Which pharmaceutical companies have the most SPCs in Ireland? appeared first on DrugPatentWatch - Make Better Decisions.

Speaker: Simran Kaur, Co-founder & CEO at Tattva.Health
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

Drug Discovery Today
MARCH 28, 2022
Phase 2 study is the first approved by the FDA and DEA to test psychedelic therapies in the area of binge eating disorders
Drug Discovery Digest brings together the best content for drug research and development professionals from the widest variety of industry thought leaders.
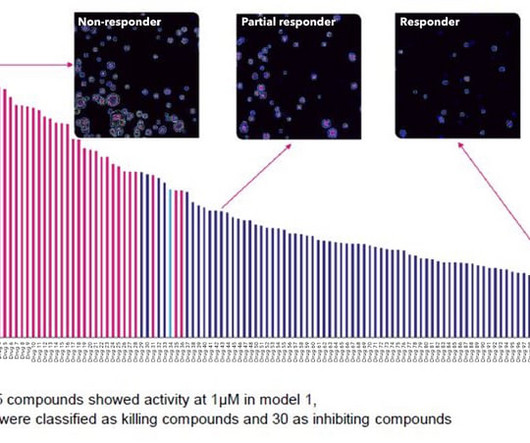
Crown Bioscience
MARCH 31, 2022
This post explores the benefits of combining high-content imaging (HCI) and high-content analysis (HCA) with 3D in vitro tumor organoids for drug discovery and validation studies. An increasing number of large in vitro screening studies are showing that novel clinically relevant biomarkers of drug response and important drug effects can be discovered by probing a wider array of cellular parameters combined with dose–response assessments.
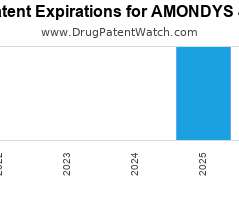
Drug Patent Watch
APRIL 1, 2022
Annual Drug Patent Expirations for AMONDYS+45 Amondys 45 is a drug marketed by Sarepta Theraps Inc and is included in one NDA. It is available from one supplier. There are…. The post New patent for Sarepta Theraps drug AMONDYS 45 appeared first on DrugPatentWatch - Make Better Decisions.

Drug Discovery Today
MARCH 28, 2022
Science Minister George Freeman says life sciences have an essential part to play in creating the economy and ecosystem of tomorrow.

Drug Channels
MARCH 28, 2022
Informa Connect’s Medicaid & Government Pricing Congress. Hybrid Event In-Person: May 23-25, 2022, Philadelphia, PA | Virtual: June 1-2, 2022 www.informaconnect.com/medicaid-government-pricing. Don’t miss the Medicaid & Government Pricing Congress , coming up May 23-25, 2022 (in-person in Philadelphia) and June 1-2, 2022 (virtually)! You'll be part of the important discussions surrounding the critical policy updates and approaches to effectively contract, report and comply with state and

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Sygnature Discovery
MARCH 31, 2022
Take a look at this new paper on drug discovery equipment
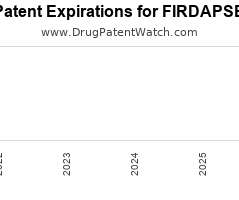
Drug Patent Watch
APRIL 1, 2022
Annual Drug Patent Expirations for FIRDAPSE Firdapse is a drug marketed by Catalyst Pharms and is included in one NDA. It is available from one supplier. There are two patents…. The post New patent for Catalyst Pharms drug FIRDAPSE appeared first on DrugPatentWatch - Make Better Decisions.

The Pharma Data
MARCH 31, 2022
Planned investments of around €2bn over next three years / Shift of resources into strategic pharmaceutical areas by investing in key production sites, manufacturing technologies and supply chain network / Bayer is transforming its pharmaceutical division through enhanced focus on breakthrough innovation as well as through consolidation and modernization of its business operations.

Drug Channels
MARCH 31, 2022
Ah, it’s finally spring in downtown Philadelphia, our home base. (Photographic evidence on your right.) Along with sunshine and fine weather, this vernal equinox has ushered in a crop of new and noteworthy stories: Health inequities in utilization management Insurers compute big white bagging savings The biosimilar boom accelerates The patient upside of manufacturers’ copay support Whoa.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Olympian Clinical Research
MARCH 31, 2022
Anxiety is the most common mental illness in the United States, affecting over 40 million adults every year. With anxiety at an all-time high as we live through a pandemic and an increasingly globalized and technologically advanced world, it can be challenging to understand what type of anxiety you may be experiencing. The most common types of anxiety are social and general anxiety, but how can you tell the difference?
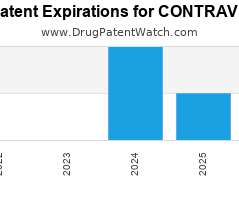
Drug Patent Watch
APRIL 1, 2022
Annual Drug Patent Expirations for CONTRAVE Contrave is a drug marketed by Nalpropion and is included in one NDA. It is available from three suppliers. There are eighteen patents protecting…. The post New patent for Nalpropion drug CONTRAVE appeared first on DrugPatentWatch - Make Better Decisions.

The Pharma Data
MARCH 31, 2022
French AMF approved EUROAPI’s listing prospectus EUROAPI first day of trading expected to occur on May 6, 2022 subject to the approval of the Distribution by the Ordinary and Extraordinary Shareholders’ Meeting to be held on May 3, 2022 Sanofi will host today a dedicated Capital Markets Day at 1:30 pm CET to present EUROAPI’s business in greater detail The Distribution ratio will be one (1) EUROAPI share per twenty three (23) Sanofi shares After the Distribution, Sanofi has confirmed its intenti
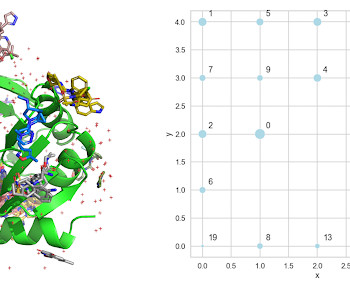
Practical Cheminformatics
MARCH 28, 2022
Clustering Fragment Screening Hits With a Self-Organizing Map (SOM) In a paper, " Fragment binding to the Nsp3 macrodomain of SARS-CoV-2 identified through crystallographic screening and computational docking ", published last year by scientists from UCSF and the Diamond Light Source, the authors reported more than 200 structures of fragments bound to the Nsp3 macrodomain of SARS-CoV-2.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

The Anticancer Fund
APRIL 1, 2022
1 April 2022 Language EN Drug repurposing is a strategy for identifying new uses for approved drugs, outside the scope of the original indication. It is one of the focus areas of the Anticancer Fund. Below, we have listed recent findings about the repurposing of generic drugs in oncology. Our intention is to help bring these findings to the attention of the broader cancer research community.
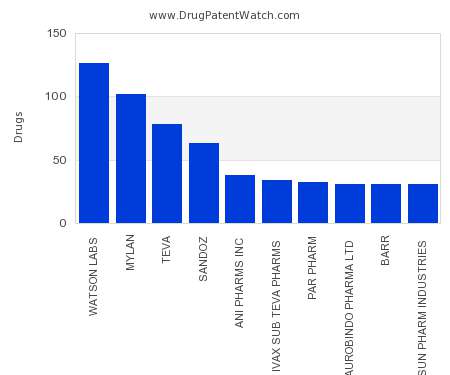
Drug Patent Watch
MARCH 31, 2022
This chart shows the pharmaceutical companies with the most capsule dosed drugs. For a different perspective, see the most popular dosage types. The companies with the most capsule dosed drugs…. The post Which pharmaceutical companies have the most capsule dosed drugs? appeared first on DrugPatentWatch - Make Better Decisions.

The Pharma Data
MARCH 31, 2022
Through Save Legs. Change Lives. ™, Janssen seeks to elevate PAD research, collaboration, education and screening for communities placed at increased risk of cardiovascular disease. The Janssen Pharmaceutical Companies of Johnson & Johnson today announced the launch of Save Legs. Change Lives.™ Spot Peripheral Artery Disease Now, a multi-year initiative aimed at creating urgency and action to address the hidden threat of peripheral artery disease (PAD)-related amputation, with an initial fo

Molecular Design
MARCH 31, 2022
Enthalpy-driven binding has been presented as a rationale for screening fragments although some have argued that thermodynamic signature is actually a 'red herring' in the context of drug discovery. Binding of a ligand grown from a fragment hit incurs a translational entropy penalty that is similar to that of the original fragment hit and it is therefore it is hardly surprising that synthetic elaboration results in binding that is more driven by entropy.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

Policy Prescription
APRIL 1, 2022
The U.S. Department of Justice, according to Seeking Alpha , is investigating the sale and distribution of counterfeit drugs in the U.S. that may have amounted to a quarter-billion dollars. The investigation comes about two months after The Wall Street Journal reported on a lawsuit filed by Gilead Sciences alleging a massive breach in the U.S. drug supply chain consisting of $250 million in counterfeit HIV drugs that were sold throughout the U.S. from licensed pharmacies to American patien
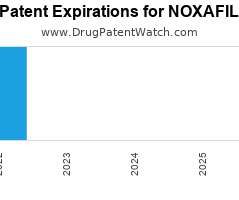
Drug Patent Watch
MARCH 31, 2022
Annual Drug Patent Expirations for NOXAFIL Noxafil is a drug marketed by Merck Sharp Dohme, Schering, and Msd Merck Co and, and is included in four NDAs. It is available…. The post New patent expiration for Schering drug NOXAFIL appeared first on DrugPatentWatch - Make Better Decisions.

The Pharma Data
MARCH 31, 2022
A Phase 2 clinical trial evaluating various additional COVID-19 booster shots has begun enrolling adult participants in the United States. The trial aims to understand if different vaccine regimens – prototype and variant vaccines alone and in combinations – can broaden immune responses in adults who already have received a primary vaccination series and a first booster shot.

Drug & Device Law
APRIL 1, 2022
Counsel defending depositions have a decision to make – whether, after opponent’s the direct examination of the witness is complete, whether to “cross-examine” a witness aligned with our own client. Usually, the answer will be “no,” because such questioning usually offers no advantages and could well undermine the witness (who may lose focus under friendly questioning) or provide clues as to the client’s trial strategy.

The Pharma Data
MARCH 31, 2022
Sanofi successfully priced yesterday, March 30, 2022, its offering of a dual-tranche EUR 1.5 billion of notes (the “Notes”). It comprises an inaugural issue of sustainability-linked bond for a nominal amount of EUR 650 million of notes, tied to Sanofi’s commitment to improve access to essential medicines in low- and lower-middle-income countries via its global health nonprofit unit.
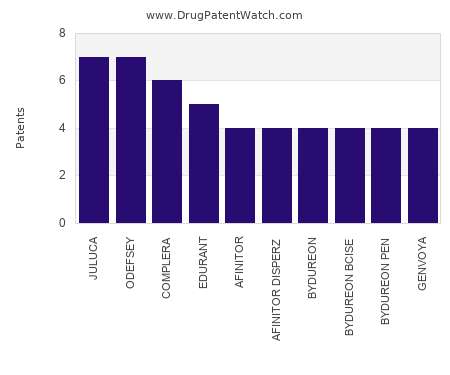
Drug Patent Watch
MARCH 30, 2022
This chart shows the drugs with the most patents in Luxembourg. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is desired.…. The post Which pharmaceutical drugs have the most drug patents in Luxembourg? appeared first on DrugPatentWatch - Make Better Decisions.
Drug Patent Watch
MARCH 30, 2022
Annual Drug Patent Expirations for KLOXXADO Kloxxado is a drug marketed by Hikma and is included in one NDA. It is available from one supplier. There are two patents protecting…. The post New patent for HIKMA drug KLOXXADO appeared first on DrugPatentWatch - Make Better Decisions.

The Pharma Data
MARCH 29, 2022
If approved, the Opdivo regimen would be the first and only immunotherapy-based option authorized for use before surgery in patients with non-small cell lung cancer ( NSCLC) in the European Union. Application based on CheckMate -816, the only Phase 3 trial to demonstrate improved event-free survival and pathologic complete response with an immunotherapy-based combination in the neoadjuvant setting of NSCLC.
Let's personalize your content