The Current issue of “The view from here” is concerned with Cancer
Drug Discovery Today
JUNE 2, 2022
The topic of this month’s newsletter from Drug Discovery Today is “Cancer”.

Drug Discovery Today
JUNE 2, 2022
The topic of this month’s newsletter from Drug Discovery Today is “Cancer”.

Drug Channels
JUNE 2, 2022
Today’s guest post comes from Bonnie Briggs, Associate Director of Clinical Effectiveness at Wolters Kluwer, Health. Bonnie describes how pharmaceutical care would benefit from standard medical record identification. She then discusses the practical issues behind implementing and adopting such a system. To learn more, download the Wolters Kluwer free ebook: Drug Data Unity: Realistic and Idealistic Futures for Information Exchange.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drug Patent Watch
MAY 30, 2022
Annual Drug Patent Expirations for VIMOVO Vimovo is a drug marketed by Horizon and is included in one NDA. It is available from one supplier. There are nine patents protecting…. The post New patent expiration for Horizon drug VIMOVO appeared first on DrugPatentWatch - Make Better Decisions.

PerkinElmer
JUNE 3, 2022
The current SARS-CoV-2 pandemic has highlighted the substantial threat posed by pathogenic coronaviruses to humans. To date, over six million people have died from COVID-19 across the globe and more than 500 million cases have been reported. 1 Although effective COVID-19 vaccines have been developed, variants of SARS-CoV-2 have already emerged for which vaccines are less effective, and many people remain unvaccinated due to certain medical conditions, personal choice, or global access challenges

Speaker: Simran Kaur, Co-founder & CEO at Tattva.Health
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

Drug Discovery Today
MAY 31, 2022
Talk from Roche engineer Tiffany McIntire will highlightopportunities for the pharma sector to reinvent packaging to bemore focused on patient needs in areas such as medication takenorally
Drug Discovery Digest brings together the best content for drug research and development professionals from the widest variety of industry thought leaders.

Drug Patent Watch
MAY 30, 2022
This chart shows the pharmaceutical companies with the most supplementary protection certificates (SPCs) in Estonia. SPCs are used in European Union and select others to encourage pharmaceutical innovation by compensating…. The post Which pharmaceutical companies have the most SPCs in Estonia? appeared first on DrugPatentWatch - Make Better Decisions.
EG Life Sciences
JUNE 2, 2022
In his last talk at Bio-IT World, Dr. Terry Barnhart spoke of the potential of Agile practices in pharmaceutical innovation. At the most recent Bio-IT World conference , Barnhart shows how Agile practices not only bring products to market faster – they also save lives. As we have discussed in previous Lean Coffees about the intersections between Agile practices and Biopharma, organizations can incorporate Agile practices without using the "Agile" label or terminology , and a major example is Ope

The Pharma Data
JUNE 2, 2022
Today, the U.S. Food and Drug Administration launched a new initiative, Supplement Your Knowledge , to help educate, inform, and broaden consumer, educator and healthcare professional understanding of dietary supplements. More than half of all Americans take dietary supplements daily or on occasion. Today’s Supplement Your Knowledge resources will provide reliable information about the potential benefits and risks associated with dietary supplements, such as vitamins, minerals, and herbs, they m

The Connected Lab
MAY 31, 2022
Digital transformation is revolutionizing pharmaceutical research and manufacturing. Driven by technologies such as advanced automation, smart sensors, artificial intelligence and machine learning, digital transformation provides new opportunities for organizations to streamline workflows, boost productivity and improve quality throughout the value chain.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Drug Patent Watch
JUNE 3, 2022
This chart shows the drugs with the most supplementary protection certificates (SPCs). SPCs are used in European Union and select others to encourage pharmaceutical innovation by compensating for the long…. The post Which drugs have supplementary protection certificates in the most countries? appeared first on DrugPatentWatch - Make Better Decisions.

The Open Targets Blog
MAY 31, 2022
Lorenzo Bomba was a postdoctoral fellow in the Soranzo group, which uses large-scale genomic analysis to study the human variation linked to cardiometabolic traits and diseases. He recently published the results of an Open Targets project which found new associations between genes and blood metabolites in the American Journal of Human Genetics.

The Pharma Data
JUNE 2, 2022
GSK plc (LSE/NYSE: GSK) announced that it has entered into a definitive agreement to acquire Affinivax, Inc. (Affinivax), a clinical-stage biopharmaceutical company based in Cambridge, Boston, Massachusetts, for a $2.1 billion upfront payment and up to $1.2 billion in potential development milestones. Affinivax is pioneering the development of a novel class of vaccines, the most advanced of which are next-generation pneumococcal vaccines.

Altus Drug Development
MAY 31, 2022
From natural health products to life-saving medicines, all pharmaceutical products must undergo an analytical method development process. Through analytical method development, validation, and transfer, drug development and manufacturing are kept safe, efficient, and compliant with the law. What is analytical method development? Analytical method development is the creation of a set of experimental conditions to perform analytical procedures in chemical samples.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
Drug Patent Watch
JUNE 3, 2022
Annual Drug Patent Expirations for JATENZO Jatenzo is a drug marketed by Clarus and is included in one NDA. It is available from one supplier. There are seven patents protecting…. The post New patent for Clarus drug JATENZO appeared first on DrugPatentWatch - Make Better Decisions.

Translation
MAY 28, 2022
Companies invest more than $2 trillion in R&D annually to stay ahead of competition, identify new products, and find new sources of revenue. These R&D efforts are fundamental to solving some of the world’s most challenging problems like combating climate change and curing diseases. Today, 80% of companies that invest in R&D partner with outside innovators.

The Pharma Data
JUNE 2, 2022
New multi-program collaboration to develop allogeneic TCR-T/CAR-T programs brings together Immatics’ allogeneic gamma delta T cell therapy platform ACTallo ® with Bristol Myers Squibb’s technologies and oncology drug development expertise. Immatics to receive upfront payment of $60 million and additional milestone payments of up to $700 million per program plus tiered royalty payments of up to low double-digit percentages on net product sales across multiple programs under the new collaboration.

Altus Drug Development
MAY 31, 2022
Value-added medicines offer the medical world the opportunity to increase the quality of treatments as well as expand access to healthcare for more people. By improving the efficiency of the healthcare system, value-based healthcare contributes to its sustainability, addresses inefficiencies related to medicines, and offers economic advantages to all parties involved in medical treatments.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

Drug Patent Watch
JUNE 2, 2022
This chart shows the pharmaceutical companies with the most supplementary protection certificates (SPCs) in Slovakia. SPCs are used in European Union and select others to encourage pharmaceutical innovation by compensating…. The post Which pharmaceutical companies have the most SPCs in Slovakia? appeared first on DrugPatentWatch - Make Better Decisions.

Broad Institute
JUNE 3, 2022
Search By admin June 3, 2022 Breadcrumb Home Search Show results from www.broadinstitute.org intranet.broadinstitute.

The Pharma Data
JUNE 2, 2022
The Phase 3 ACTIV-1 Immune Modulators study was sponsored by the National Institutes of Health as part of the ACTIV initiative. Orencia was one of two immune modulators that improved survival for people hospitalized with COVID-19. Safety profile of Orencia remained consistent, with no new safety signals reported. Bristol Myers Squibb (NYSE: BMY) today announced topline results from the Phase 3 Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV-1) Immune Modulators clinical tri

Altus Drug Development
MAY 31, 2022
Everyone is interested in having access to the best healthcare available. However, many patients end up having to face the fact that many treatments are unfeasible, too expensive, or potentially counter-conducive. Knowledge of issues within the healthcare system is not unknown to those in charge. However, dealing with them often requires making structural changes many industry players are not willing or able to perform.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic
Drug Patent Watch
MAY 31, 2022
Annual Drug Patent Expirations for OLUMIANT Olumiant is a drug marketed by Eli Lilly And Co and is included in one NDA. It is available from one supplier. There are…. The post New patent for Eli Lilly drug OLUMIANT appeared first on DrugPatentWatch - Make Better Decisions.

National Drug & Alcohol Research Centre Blog
MAY 30, 2022
Dr Ryan Courtney and Jack Anderson Tobacco smoking is the leading preventable cause of death, globally. In 2019, approximately 14% of Australians aged 14 years or older were current smokers. That’s approximately 2.9 million Australians. On World No Tobacco Day , Dr. Ryan Courtney and his team would like to highlight the importance of quit smoking research in low socioeconomic populations and provide details on an exciting new technology-based quit support study that begins recruitment in June 20

The Pharma Data
JUNE 2, 2022
BYOOVIZ™ is the first FDA approved ophthalmology biosimilar BYOOVIZ, priced 40% lower than LUCENTIS®, provides an equally effective and more affordable treatment option to patients suffering from retinal disorders BYOOVIZ will be commercially available through major distributors across the U.S. on July 1, 2022. Biogen Inc. (Nasdaq: BIIB) and Samsung Bioepis Co., Ltd. today announced that BYOOVIZ™ (ranibizumab-nuna), a biosimilar referencing LUCENTIS® (ranibizumab) i has been launched in the Un

Altus Drug Development
MAY 31, 2022
Thanks to oral formulation, many drugs crucial for the health and well-being of millions of people can be administered and save countless lives. The field of drug formulation is in a constant state of innovation, creating a plethora of challenges to overcome, but also opening up a world of opportunities. What is oral formulation? Oral formulation is the development and manufacturing of pharmaceuticals designed for oral delivery.
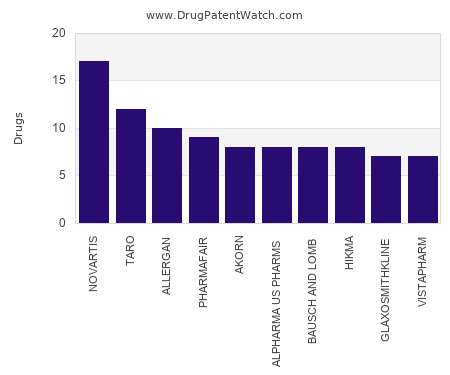
Drug Patent Watch
MAY 31, 2022
This chart shows the pharmaceutical companies with the most suspension dosed drugs. For a different perspective, see the most popular dosage types. The companies with the most suspension dosed drugs…. The post Which pharmaceutical companies have the most suspension dosed drugs? appeared first on DrugPatentWatch - Make Better Decisions.
Let's personalize your content