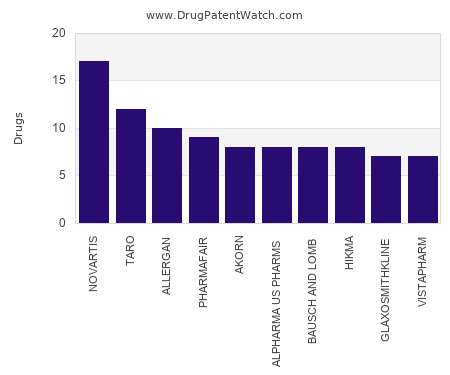
Patient centricity in drug innovation and packaging design to take centre stage at Connect in Pharma in Geneva this September
Drug Discovery Today
MAY 31, 2022
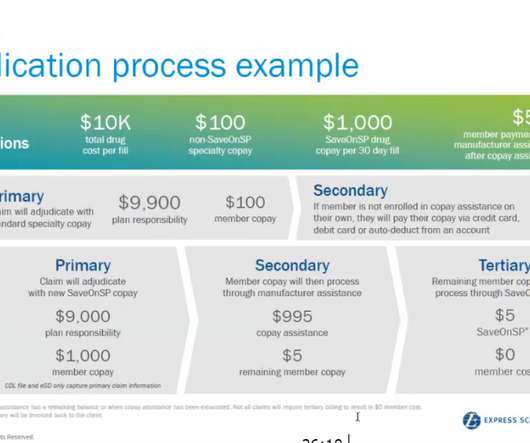
Talk from Roche engineer Tiffany McIntire will highlightopportunities for the pharma sector to reinvent packaging to bemore focused on patient needs in areas such as medication takenorally





































Let's personalize your content