Getting Ozempic And Other Drugs From Canada? Not Likely.
Forbes: Drug Truths
APRIL 6, 2023
Clearly, Canada would find such a situation untenable and the steps now being taken by British Columbia for Ozempic would be adopted across Canada.

Forbes: Drug Truths
APRIL 6, 2023
Clearly, Canada would find such a situation untenable and the steps now being taken by British Columbia for Ozempic would be adopted across Canada.

Chemical Biology and Drug Design
APRIL 7, 2023
Carbon–silicon bioisosteric switch as a strategy to modulate drug-like properties of anticancer agents: molecular design, biological activity modulation, computational modeling, and structure–activity relationship studies. Abstract Bioisosterism is one of the leading strategies in medicinal chemistry for the design and modification of drugs, consisting in replacing an atom or a substituent with a different atom or a group with similar chemical properties and an inherent biocompatibility.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Antidote
APRIL 6, 2023
At any given time, there are hundreds of thousands of research studies listed on ClinicalTrials.gov actively looking for patients both in the United States and across the globe. However, the multitude of options available does not necessarily mean it’s easy for interested individuals to find opportunities they are eligible for. That’s why we created Antidote Match™, a clinical trial search tool that allows users to answer a few questions about their health and find clinical trial listings that c

Drug Patent Watch
APRIL 6, 2023
This chart shows the pharmaceutical companies with the most supplementary protection certificates (SPCs) in Netherlands. SPCs are used in European Union and select others to encourage pharmaceutical innovation by compensating… The post Which pharmaceutical companies have the most SPCs in Netherlands? appeared first on DrugPatentWatch - Make Better Decisions.

Speaker: Simran Kaur, Co-founder & CEO at Tattva.Health
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

Drug Channels
APRIL 7, 2023
Today’s guest post comes from Heather Roulston, Market Research Manager at MMIT. Using MMIT’s proprietary data, Heather demonstrates that contracting for medical benefit drugs is now common for many therapeutic areas. She then highlights how manufacturers and payers are adopting value-based agreements (VBA) for medical benefit products—although VBA usage varies widely across therapeutic area.
Drug Discovery Digest brings together the best content for drug research and development professionals from the widest variety of industry thought leaders.

ProRelix Research
APRIL 5, 2023
Clinical trials form the decisions for regulatory approval of drugs and medical devices and as a result are the most important step of a drug development program. In addition to […] The post Investigator Initiated Trials (IITs): Introduction, regulatory view, study design preferences, and p … appeared first on ProRelix Research.

NIOSH Science Blog: Drugs
APRIL 4, 2023
Struck-By Injuries Struck-by injuries occur from violent contact or impact between an object or piece of equipment and a person. Struck-by injuries can be fatal, and even when a worker is not seriously injured can result in days off work to recover. To help prevent struck-by injuries, companies are encouraged to have a stand-down; a voluntary event for employers to talk directly to employees about safety.

Drug Channels
APRIL 4, 2023
Time for Drug Channels Institute’s annual update on the gross-to-net bubble —the ever-growing dollar gap between sales at brand-name drugs' list prices and their sales at net prices after rebates and other reductions. We estimate that the gross-to-net bubble reached $223 billion for patent-protected brand-name drugs in 2022. If we include brand-name drugs that have lost patent protection and face competition from generic equivalents, the bubble was even higher, at $256 billion.

Chemical Biology and Drug Design
APRIL 3, 2023
Eight derivatives of benzyloxy-derived halogenated chalcones ( BB1-BB8 ) were synthesized and tested for their ability to inhibit monoamine oxidases (MAOs). Abstract Eight derivatives of benzyloxy-derived halogenated chalcones ( BB1-BB8 ) were synthesized and tested for their ability to inhibit monoamine oxidases (MAOs). MAO-A was less efficiently inhibited by all compounds than MAO-B.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

National Institute on Drug Abuse: Nora's Blog
APRIL 5, 2023
Creating Sustainable Homes for Prevention Services mfleming Wed, 04/05/2023 - 12:12 Nora's Blog April 12, 2023 Image ©Getty Images/ Willie B. Thomas The addiction and overdose crisis in the U.S. continues unabated, with more than 46 million people having a substance use disorder (SUD) in 2021 and more than 100,000 people dying from drug overdose annually.

FDA Law Blog: Biosimilars
APRIL 3, 2023
By Alan M. Kirschenbaum — On March 31, the Federal District Court for the District of Maryland upheld CMS’s definition of a “new formulation” under the Medicaid Drug Rebate Program (MDRP). Vanda Pharmaceuticals, Inc. v. CMS, Civ. No. MJM-22-977 (Dist. Md. 2023). By way of background, manufacturers are subject to an additional per-unit Medicaid rebate if they increase their prices greater than the rate of inflation.

NIOSH Science Blog: Drugs
APRIL 7, 2023
Camilla started experiencing worsening respiratory symptoms while working at a plant that produces greeting cards. (Read about her symptoms here). Her doctor ordered tests to see what was happening with Camilla’s lungs. The results of two lung function tests showed concerning results. One was a carbon monoxide diffusing capacity test that estimates the ability of the lung to transfer oxygen from the air to the person’s bloodstream.

Chemical Biology and Drug Design
APRIL 2, 2023
TFP5 treatment inhibited the overexpression of CDK5 in high glucose environment, reduced the inflammatory response, oxidative stress, and apoptosis of islet β cells. Abstract Islet β-cell damage and dysfunction represent the pathophysiological basis of diabetes. Excessive activation of cyclin-dependent kinase 5 (CDK5) is involved in the pathogenesis of type 2 diabetes mellitus (T2DM), although the exact mechanism remains unclear.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Precision for Medicine
APRIL 7, 2023
Houston is one of seven global Precision specialty labs addressing the rising demand for rapid, comprehensive biomarker data to support the development of advanced therapies and diagnostics HOUSTON, TX, April 12, 2023 – Precision for Medicine, the first global biomarker-driven clinical research organization, today announced the expansion of its cell biology and genomics laboratory in Houston, TX, proliferating the company’s global biomarker capabilities and laboratory operations.
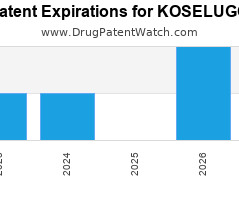
Drug Patent Watch
APRIL 7, 2023
Annual Drug Patent Expirations for KOSELUGO Koselugo is a drug marketed by Astrazeneca and is included in one NDA. It is available from one supplier. There are four patents protecting… The post New patent for Astrazeneca drug KOSELUGO appeared first on DrugPatentWatch - Make Better Decisions.

NIOSH Science Blog: Drugs
APRIL 3, 2023
Camilla works at a plant that produces greeting cards and ribbon products. She started working at the plant over 15 years ago when production first started. The plant is open around-the-clock for three shifts with workers in multiple departments across two floors of the building. Camilla worked in different departments over the years but spent the most time on the morning shift in greeting card production.

Chemical Biology and Drug Design
APRIL 1, 2023
Berberine has been shown in in vitro and in vivo studies to inhibit various types of cancer by activating the p-53 and cyclin-B expression, targeting various cell signalling pathways, to regulate the cell cycle. It also inhibits the invasion and metastasis of cancer cells. Besides this, it induces cell death by apoptosis, autophagy and necrosis in cancer cells.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

On Medicine
APRIL 6, 2023
Note from Prof. Steven Lipshultz, Editor-in-Chief of Cardio-Oncology , and Miriam Mestre, Managing Editor. This inaugural conference was jointly sponsored by Sofia’s Hope, the Cincinnati Children’s Heart Institute, and Oishei Children’s Hospital/Jacobs School of Medicine and Biomedical Sciences, University at Buffalo. The “ Cardio-Oncology Journal Prize” for top original research posters was sponsored by BMC.
Drug Patent Watch
APRIL 7, 2023
Annual Drug Patent Expirations for TAZVERIK Tazverik is a drug marketed by Epizyme Inc and is included in one NDA. It is available from one supplier. There are twenty-four patents… The post New patent for Epizyme Inc drug TAZVERIK appeared first on DrugPatentWatch - Make Better Decisions.

Cyclica
APRIL 6, 2023
As a proud “working mom,” Jen is the VP of Marketing and Communications at Cyclica. Prior to joining Cyclica, she spent 6 years working in Zürich, Switzerland, in marketing communications based roles within the biotech and tech sectors, including having led European Oncology and R&D communications at Amgen Europe. While always maintaining an active lifestyle by running, cycling, weight training, and yoga, living near the alps facilitated a newfound passion for hiking.

Cytel
APRIL 5, 2023
Health technology assessment (HTA) submissions require cost effectiveness analyses based on comparative effectiveness studies of survival benefits vs. standard-of-care options in each specific geography. Ideally, these submissions are based on large, randomized control trials (RCTs), however, most new drugs approved in the last five years, specifically in oncology and rare diseases, are being approved based on small clinical trials, often un-controlled or single arm.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

H1 Blog
APRIL 5, 2023
For clinical operations and development clinical feasibility teams, diversity equity and inclusion and health equity leaders, as well as health economics and outcomes research (HEOR) teams, it is essential to understand the considerations when integrating data to help ensure inclusive and equitable compliant clinical trials that increase trust among patients, pharma, and study participants.
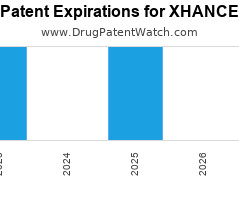
Drug Patent Watch
APRIL 6, 2023
Annual Drug Patent Expirations for XHANCE Xhance is a drug marketed by Optinose Us Inc and is included in one NDA. It is available from one supplier. There are thirteen… The post New patent for Optinose Us drug XHANCE appeared first on DrugPatentWatch - Make Better Decisions.

BMG Labtech
APRIL 5, 2023
The importance of drug solubility Today’s drug discovery environment demands efficiency, performance and safety. A crucial part of the process is the ability to perform ADMET (absorption, distribution, metabolism , elimination and toxicity) assays as early as possible in the drug development process. For example, researchers need to know quickly if a drug does not have the right toxicity profile before investing further resources and time in advancing it to preclinical and clinical testing.

Cyclica
APRIL 5, 2023
Maya Angelou once said — 'I've learned that people will forget what you said , people will forget what you did , but people will never forget how you made them feel.

BenchSci
APRIL 5, 2023
For our first Scale Up talk of the year, we hosted Luba Greenwood. Luba gracefully agreed to join us today to talk to us about her experience as a seasoned biotech, tech, pharmaceutical, and life sciences investor as well as a company builder. Luba’s career has taken her through multiple fields, including practicing law at WilmerHale, leading Business Development at Verily (Google), and serving as Vice President of Global Mergers & Acquisitions and Business Development at Roche.
Let's personalize your content