Upcoming Webinar: Artificial Intelligence Drug Discovery — Where are we now?
Collaborative Drug
MAY 1, 2023
Upcoming Webinar: Artificial Intelligence Drug Discovery — Where are we now?

Collaborative Drug
MAY 1, 2023
Upcoming Webinar: Artificial Intelligence Drug Discovery — Where are we now?
Covalent Modifiers
MAY 3, 2023
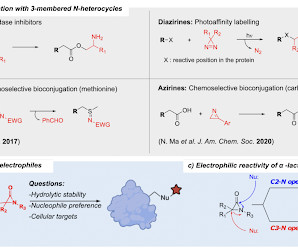
Mahía, Alejandro , Kiib, Anders , Nisavíc, Marija , Svenningsen, Esben , Palmfeldt, Johan , Poulsen, Thomas Bjørnskov , Angew. Chem. Int. Ed. 2023 , e202304142 [link] Electrophilic groups are one of the key pillars of contemporary chemical biology and medicinal chemistry. For instance, the group of 3-membered N-heterocyclic compounds – such as aziridines, azirines, and oxaziridines – possess unique electronic and structural properties which underlie their potential and applicability as covalent
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drug Discovery Today
MAY 4, 2023
London, UK, 3rd May 2023 / The RSA Group, a leading life sciences executive search firm, has published the latest of its Talent Equity® Reports: No turning back – an analysis of the hybrid working models that have emerged from the pandemic and how they have enabled positive changes to leadership, team dynamics and recruitment and retention of talent in the life science sector.

ASPET
MAY 4, 2023
Drug biliary clearance (CL bile ) in vivo is among the most difficult pharmacokinetic parameters to predict accurately and quantitatively because biliary excretion is influenced by metabolic enzymes, transporters, and passive diffusion across hepatocyte membranes. The purpose of this study is to demonstrate the use of Hu-FRG{trade mark, serif} mice (Fah –/– /Rag2 –/– /Il2rg –/– [FRG] mice transplanted with human-derived hepatocytes) to quantitatively predict h

Speaker: Simran Kaur, Co-founder & CEO at Tattva.Health
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

Collaborative Drug
MAY 4, 2023
Drug Discovery Industry Roundup with Barry Bunin — May 3, 2023
Drug Discovery Digest brings together the best content for drug research and development professionals from the widest variety of industry thought leaders.

Chemical Biology and Drug Design
MAY 1, 2023
We found overexpression of C-FLIPS, MCL-1, and survivin by SphK2 silencing has an effective important modality to enhance cell sensitivity to paclitaxel via the induction of apoptosis in colon cancer cells. Abstract The sphingosine kinase-2 (SphK2), a main component of sphingolipid signal transduction, is reported as an innovative therapeutic candidate for cancer treatment.

Antidote
MAY 3, 2023
Every May is dedicated to Skin Cancer Awareness Month — a national observance designed to raise awareness around skin cancer and encourage people to practice smart prevention tactics as spring transitions into summer. Though skin cancer is the most common type of cancer , forming good sun safety habits can have a direct link to a reduction in its prevalence.

Drug Patent Watch
APRIL 30, 2023
Annual Drug Patent Expirations for AGGRASTAT Aggrastat is a drug marketed by Medicure and is included in two NDAs. It is available from one supplier. There is one patent protecting… The post New patent expiration for Medicure drug AGGRASTAT appeared first on DrugPatentWatch - Make Better Decisions.

Drug Channels
MAY 2, 2023
In my most recent video webinar , I explored how the rapid expansion of patient-paid prescriptions—via cash-pay pharmacies and discount card vendors—is transforming the prescription market. Below, I follow the dollar when a patient uses a discount card to pay for a generic drug prescription. As you’ll see, a discount card can save money for consumers by leveraging several quirks of U.S. retail pharmacy pricing.

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven

Chemical Biology and Drug Design
APRIL 29, 2023
MYC regulates main genes of exosome biogenesis in breast cancer. Furthermore, MYC regulates oncogenic roles of breast cancer cells-derived exosomes in breast cancer and MYC-expressing cancer cells-secreted exosomes induce higher cellular activity of recipient cells in breast cancer. Abstract MYC amplification and overexpression in breast cancer occur 16% and 22%, respectively, and MYC has a linchpin role in breast carcinogenesis.

FDA Law Blog: Drug Discovery
MAY 4, 2023
By McKenzie E. Cato — On May 2nd, FDA released a new draft guidance with recommendations for decentralized clinical trials (DCTs) for drugs, biologics, and devices. A DCT is a clinical trial where some or all of the trial-related activities, such as administration of the investigational product, data collection, or safety monitoring, occur at locations other than traditional clinical trial sites.
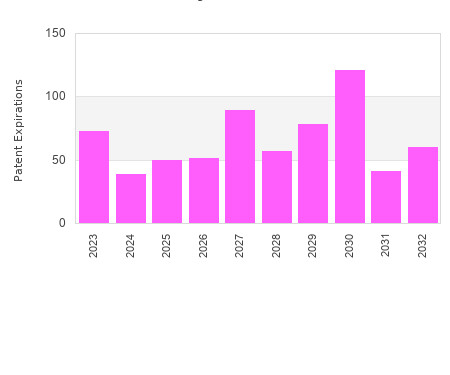
Drug Patent Watch
MAY 2, 2023
This chart shows the patent expirations for capsule dosed drugs over the next decade. The term of drug patents varies. The basic term for a patent is 20 years from… The post Capsule dosed drug patent expirations by year appeared first on DrugPatentWatch - Make Better Decisions.

FDA Law Blog: Biosimilars
MAY 3, 2023
By Deborah L. Livornese — FDA posted the last five “deemed final orders” under OTC monograph reform this week. All 33 of the final orders can now be found at OTCMONOGRAPHS@FDA. Under the 2020 Coronavirus Aid, Relief, and Economic Security Act (CARES Act) (see our blog post here ), all final monographs (i.e., the regulations formerly found in 21 C.F.R. parts 331 through 358) were deemed to be final administrative orders as of the CARES Act effective date.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

Cytel
MAY 5, 2023
As clinical trials become more complex, simulation-guided design approaches are crucial. For this edition of the Industry Voices series, Cytel’s Chief Product Officer & General Manager Scott Gaines discusses how simulation-guided design and Cytel’s Solara® clinical strategy platform have evolved clinical development strategy, enabling sponsors to select optimal designs even as clinical trials involve more study objectives and more sophisticated decision rules, and to better focus on strategi

Reprocell
MAY 5, 2023
Members of the UK Parliament were impressed and somewhat surprised recently by an exchange between the Prime Minister and the leader of the opposition during Prime Minister’s questions. 1 The opponents exchanged polite introductions and proceeded to debate the issues of the day in a calm and thoughtful manner, without raising their voice. The members were in fact watching an exchange with an AI chatbot, programmed to answer questions of the type often raised in the weekly shouting match.

Drug Target Review
MAY 5, 2023
Personalised Precision Medicine (PPM) relies heavily on the use of data and artificial intelligence (AI) to tailor treatment plans to an individual’s unique biomarkers , predict their reaction to certain treatments, and target diseases based on their genetic makeup or environmental factors. While PPM holds great promise for improving patient outcomes, it also faces challenges such as data management, security and the escalating costs of research and development.

FDA Law Blog: Biosimilars
APRIL 30, 2023
By Kurt R. Karst & Michael D. Shumsky — On May 2nd, the U.S. Senate Committee on Health, Education, Labor and Pensions (“Senate HELP”) is scheduled to take up legislation that could significantly limit access to the courts and immunize critical FDA decisions from timely judicial review. That bill is S. 1067, the “ Ensuring Timely Access to Generics Act of 2023 ,” and it would fundamentally transform the playing field for NDA, ANDA, BLA, and aBLA applicants seeking to preserve their rights i

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.
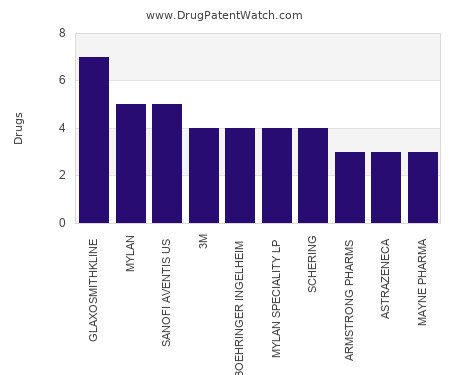
Drug Patent Watch
MAY 5, 2023
This chart shows the pharmaceutical companies with the most aerosol dosed drugs. For a different perspective, see the most popular dosage types. The companies with the most aerosol dosed drugs… The post Which pharmaceutical companies have the most aerosol dosed drugs? appeared first on DrugPatentWatch - Make Better Decisions.

Antidote
MAY 5, 2023
Patient Experience Week is a designated time to acknowledge the healthcare staff that influence the patient experience every single day. From doctors and nurses to site staff and research coordinators, there are many individuals in the healthcare industry who make a difference in the lives of patients.

The Premier Consulting Blog
MAY 4, 2023
The global ophthalmic drugs market size was valued at $33.81 billion in 2022 and is expected to grow approximately eight percent from 2023 to 2030 [1]. Given this trend, many biopharmaceutical companies are investing in strategies to develop and commercialize new or existing ophthalmic products that address unmet medical needs. Now, these companies are required to comply with new guidance from the U.S.

PLOS: DNA Science
MAY 4, 2023
Dense living communities of hundreds of bacterial species form biofilms on our teeth. Without careful brushing and flossing of this dental plaque, minerals seep in, hardening it into tartar. When proteins in saliva adhere tartar to tooth surfaces, a trip to the dentist is required to hack the stuff off. Over time, the mineralized microbes of tooth tartar come to comprise a mouthful of tiny fossils, including snippets of degraded bacterial DNA.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic
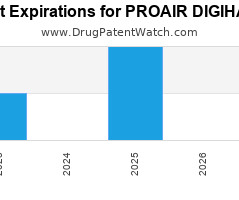
Drug Patent Watch
MAY 5, 2023
Annual Drug Patent Expirations for PROAIR+DIGIHALER Proair Digihaler is a drug marketed by Teva Branded Pharm and is included in one NDA. It is available from one supplier. There are… The post New patent expiration for Teva Branded drug PROAIR DIGIHALER appeared first on DrugPatentWatch - Make Better Decisions.

H1 Blog
MAY 4, 2023
Insights for Thought Leading Stakeholder Engagement to Decrease Medication Non-Adherence Pharmaceutical companies have long recognized the importance of engaging key opinion leaders (KOLs) to gain insight and support for clinical research, drug development, and medical education. With the rise of digital technology, pharma can now identify and engage a new generation of health care professionals (HCPs) who are leveraging social media to become influential digital opinion leaders.

addgene Blog
MAY 4, 2023
The biomedical field is often concerned with understanding the cause of diseases and how to treat those diseases. The “cause of disease” often requires understanding the disease genetics and the “treatment” usually requires drugs. While we often think of these two fields as separate, they are deeply intertwined. In this blog we will review how CRISPR technology has brought drug and genetic screens together and expedited the drug discovery pipeline.

DS in Pharmatics
MAY 4, 2023
Diagnosing illnesses and monitoring health is increasingly reliant on utilizing software. For patients, manufacturers must maintain standards and regulations. Companies can be concerned with challenges concerning updated legislation such as the EU’s MDR.
Drug Patent Watch
MAY 5, 2023
Annual Drug Patent Expirations for PROAIR+RESPICLICK Proair Respiclick is a drug marketed by Teva Branded Pharm and is included in one NDA. It is available from one supplier. There are… The post New patent expiration for Teva Branded drug PROAIR RESPICLICK appeared first on DrugPatentWatch - Make Better Decisions.
Let's personalize your content