CDD Exhibiting and Presenting at Bio-IT World, Boston, May 16-18, 2023
Collaborative Drug
MAY 12, 2023
CDD Exhibiting and Presenting at Bio-IT World, Boston, May 16-18, 2023

Collaborative Drug
MAY 12, 2023
CDD Exhibiting and Presenting at Bio-IT World, Boston, May 16-18, 2023
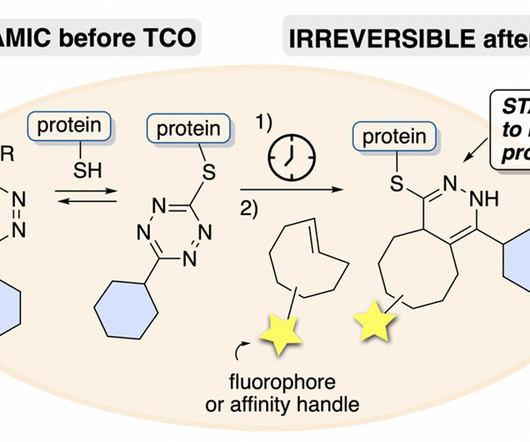
Covalent Modifiers
MAY 11, 2023
Amanda Tallon, Yingrong Xu, Graham West, Christopher am Ende, Joseph Fox ChemRXiv , 2023 [link] Electrophilic small molecules that can reversibly modify proteins are of growing interest in drug discovery. However, the ability to study reversible covalent probes in live cells can be limited by their reversible reactivity after cell lysis and in proteomic workflows, leading to scrambling and signal loss.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
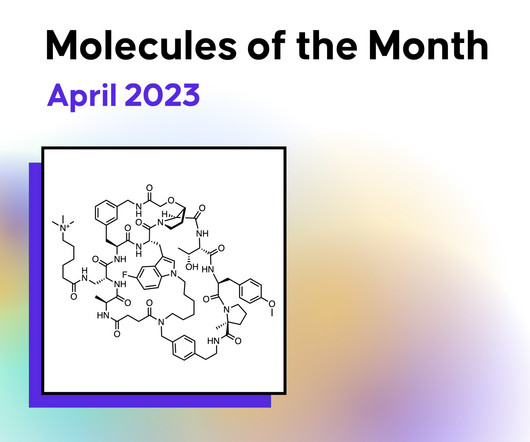
Drug Hunter
MAY 11, 2023
An oral macrocyclic peptide PCSK9 inhibitor entering Ph. III for hypercholesterolemia later this year, a Ph. I/Ib KRAS G12C (ON) tri-complex inhibitor for the treatment of advanced solid tumors, an oral mutant-selective BRAFC600X degrader in Ph. I for BRAF-driven cancers, and an orally available AR-degrading PROTAC in Ph. II for advanced prostate cancers are just some of the examples for this month’s MOTM.

Chemical Biology and Drug Design
MAY 11, 2023
A series of [5,5′-bibenzo[d][1,3]dioxol]-6-amine analogs were designed and synthesized based on our previous work. Compounds D28 and D29 impaired PCSK9/LDLR PPI, restored LDLR expression, and improved extracellular LDL uptake of HepG2 cells in the presence of PCSK9. Abstract Proprotein convertase subtilisin/kexin type 9 (PCSK9) has emerged as a promising therapeutic target for the treatment of hyperlipidemia.

Speaker: Simran Kaur, Co-founder & CEO at Tattva.Health
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

ASPET
MAY 10, 2023
Irritable bowel syndrome (IBS) and bladder pain syndrome/interstitial cystitis (BPS/IC) are comorbid visceral pain disorders seen commonly in women with unknown etiology, limited treatment options and can involve visceral organ cross-sensitization. Calcitonin gene-related peptide (CGRP) is a mediator of nociceptive processing and may serve as a target for therapy.
Drug Discovery Digest brings together the best content for drug research and development professionals from the widest variety of industry thought leaders.

Antidote
MAY 10, 2023
One of the questions most frequently asked by patients is, “Do I have to pay to participate in a clinical trial?” While patients typically will not incur expenses for taking part in a research study, in some instances, they may be responsible for copays and payments towards their deductible depending on their insurance plan.

Chemical Biology and Drug Design
MAY 11, 2023
Certain Plasmodium falciparum autophagy-related proteins ( Pf Atg) play autophagic and/or non-autophagic functions. Also, Pf Atg8 was found to have unique hydrophobic pockets, which it uses for interaction with other proteins. Moreover, the most formidable inhibitor of Pf Atg8- Pf Atg3 PPI was discovered to be 2-bromo-N-(4-pyridin-2-yl-1,3-thiazol-2-yl) benzamide.

Drug Channels
MAY 10, 2023
Yesterday’s reflections on Asembia’s Specialty Pharmacy Summit included a link to the general session slides. My portion of the deck included an updated mapping of vertical integration among insurers, PBMs, specialty pharmacies, and providers within U.S. drug channels. Many readers asked for a standalone version of this infamous chart. So, for your viewing and slide making pleasure, below you’ll find my latest illustration of the major vertical business relationships among the largest companies.

FDA Law Blog: Biosimilars
MAY 8, 2023
By Lisa M. Baumhardt, Senior Medical Device Regulation Expert & Philip Won & Gail H. Javitt — FDA recently published a long-awaited draft guidance aimed at reducing the need for prior FDA authorization of modifications to artificial intelligence/machine learning (AI/ML)-enabled device software functions (ML-DSFs). The draft guidance, “Marketing Submission Recommendations for a Predetermined Change Control Plan for Artificial Intelligence/Machine Learning (AI/ML)-Enabled Device Software

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven
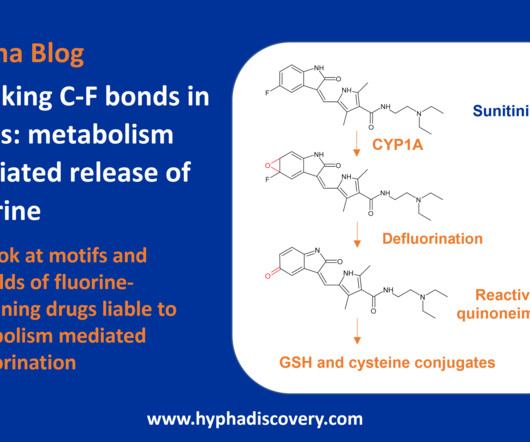
Metabolite Tales Blog
MAY 12, 2023
Breaking C-F bonds in drugs – metabolism mediated release of fluorine By Samuel Coe and Julia Shanu-Wilson Lenacapavir, recently approved for multi-drug resistant HIV-1 infection, contains 10 fluorine atoms. Fluorine is a highly reactive chemical element that has a unique electron configuration and provides valuable benefits in medicinal chemistry.

Chemical Biology and Drug Design
MAY 11, 2023
In-vitro and in-vivo anti-leishmanial activity of the hanging sedge flavonoids; based on bio- guided fractionation assay. Abstract As a major public health issue, cutaneous leishmaniasis (CL) has a number of complications, including drug resistance and poor response to conventional treatments. Over the last decade, research on natural sources for finding new antileishmanial agents has been a critical part of tropical disease research.

Drug Channels
MAY 9, 2023
Last week, Paula and I had the pleasure of attending Asembia’s 2023 Specialty Pharmacy Summit at the wonderful Wynn Las Vegas. As I do every year, I will violate Vegas code and tell you what happened there. I offer reflections on my keynote session with Seema Verma, share my experiences during the featured session, and highlight some crucial specialty industry trends.

FDA Law Blog: Biosimilars
MAY 11, 2023
By Sara W. Koblitz — Well, we’re a little late to blogging about this, but the significance of the ongoing Teva v. GSK litigation to Hatch-Waxman aficionados makes this case still ripe for blogging. Six months after the Supreme Court asked the Solicitor General to submit a brief on behalf of the U.S. government in the now-infamous (at least in FDA circles) Teva v.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.

biobide
MAY 12, 2023
Schizophrenia is a chronic psychiatric disorder characterized by continuous or relapsing episodes of psychosis that can alter severely normal brain function. This mental health issue makes it arduous to determine what is real or not, producing symptoms such as hallucinations, delusions, and disorganized thinking. Therefore, this illness is a serious mental disorder that affects around 1 to 300 people, meaning 24 million people globally.

Chemical Biology and Drug Design
MAY 11, 2023
We designed an fusion protein by connecting a cetuximab-derived single-chain variable fragment targeting the epidermal growth factor receptor (anti-EGFR scFv) and the anticancer lytic peptide (ACLP) ZXR2. The results suggest that scFv-ACLP fusion proteins may be potential anticancer drug candidates for targeted cancer treatment. Abstract Antibody-directed drugs for targeted cancer treatment have become a hot topic in new anticancer drug development; however, antibody-fused therapeutic peptides w
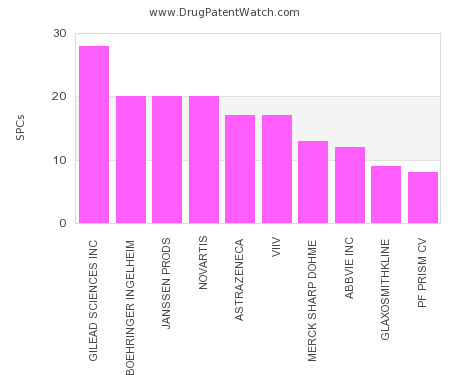
Drug Patent Watch
MAY 12, 2023
This chart shows the pharmaceutical companies with the most supplementary protection certificates (SPCs) in Lithuania. SPCs are used in European Union and select others to encourage pharmaceutical innovation by compensating… The post Which pharmaceutical companies have the most SPCs in Lithuania? appeared first on DrugPatentWatch - Make Better Decisions.

FDA Law Blog: Biosimilars
MAY 9, 2023
By Jeffrey N. Wasserstein — We were listening to a radio interview last week with Lina Khan , the Chair of the Federal Trade Commission (“FTC”). In the interview, Khan spoke about the Commission’s efforts to regulate geolocation data trackers so that they don’t abuse their abilities. The risks she described caught our attention because two of the risk profiles she cited included things that touched on FDA-related entities, such as medical devices that transmit data.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.

Drug Channels
MAY 12, 2023
Today’s guest post comes from Nasir Ali, Chief Product Officer at CareMetx Nasir discusses manufacturers’ strategies for mitigating the patient impact of copay accumulators and maximizers. He then outlines two key tactics that manufacturers should include in hub programs. To learn more how digitally-enabled hub programs help patients, download CareMetx’s report: The Evolving Landscape of Digital Healthcare Hubs.

Chemical Biology and Drug Design
MAY 11, 2023
New analogs of dual-target MOR/DOR ligand LP2 were synthesized. In-vitro, their affinity profile versus opioid receptors was performed and molecular modeling studies were conducted to deeply analyze the binding mode and the interactions between the new ligands and all opioid receptors. Abstract 6,7-Benzomorphans have been investigated in medicinal chemistry for developing new drugs.

Drug Patent Watch
MAY 12, 2023
Annual Drug Patent Expirations for VASOSTRICT Vasostrict is a drug marketed by Par Sterile Products and is included in one NDA. It is available from six suppliers. There are fourteen… The post New patent for Par Sterile drug VASOSTRICT appeared first on DrugPatentWatch - Make Better Decisions.

Codon
MAY 11, 2023
Hey there. Tap tap tap. Today, we’ve got news about AI mind readers, a child who underwent brain surgery while in the womb, and (another) problem with lab-grown meat. Scroll to the end for a beautiful chart about the Anthropocene & some details on insect engineering companies. Fun. Also. news items are now sent on Thursdays. Research roundups are sent on Sundays.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic

The Premier Consulting Blog
MAY 11, 2023
In this second of our two-part series, we continue our discussion about significant recent developments regarding the regulation of ophthalmic products and discuss what to expect in the months ahead. Unsurprisingly, the U.S. Food and Drug Administration’s (FDA) released updated guidance following the landmark Genus Medical Technologies LLC v. United States Food and Drug Administration court decision.

Chemical Biology and Drug Design
MAY 11, 2023
Synthesized analogs MMA307 and MMA320 induce G1 phase cell cycle arrest and inhibit EGFR-MAPK Proteins involved in tumor progression in non-small-cell lung cancer. Molecular dynamic simulations provided additional confirmation of biological activity. Abstract Lung cancer is the deadliest human cancer globally, with non-small-cell lung cancer (NSCLC) being the most frequent type.

thought leadership
MAY 11, 2023
Our "Meet the Expert" series introduces you to our team of experts around the world. This "behind the curtain" view will help you get to know who we are on a professional and personal level, and highlight how our colleagues work together on our higher purpose to improve patient health and safety throughout the complete product lifecycle.

On Medicine
MAY 11, 2023
There are a few (but growing) centers that will proactively see patients with diabetes in high-risk areas when admitted to the hospital, however, by far the most common practice is for patients to only be seen by a specialist team, including for diabetes, on receipt of a referral. In diabetes, referrals are often triggered by glycemic-related events, such as hypo/hyperglycemia and ketosis or for patient education.

PPD
MAY 11, 2023
The global COVID-19 pandemic increased awareness of the importance of vaccine development — both for drug developers and the public. The speed at which COVID-19 vaccines were developed was remarkable, but like most newly developed vaccines, there was variation among who could receive the shots and when. To effectively implement vaccine clinical trials for special populations, it is critical for vaccine developers to partner with a contract research organization (CRO) that has demonstrated
Let's personalize your content