Enhanced pacemaker developer Ceryx Medical wins prestigious Institute of Physics award
Drug Discovery Today
NOVEMBER 3, 2022
University of Bath spin-out hailed for revolutionary development to provide world’s first curative therapy for heart failure

Drug Discovery Today
NOVEMBER 3, 2022
University of Bath spin-out hailed for revolutionary development to provide world’s first curative therapy for heart failure

Drug Channels
NOVEMBER 4, 2022
Today’s guest post comes from Matthew Balogh, Head of Digital Marketing at Melinta Therapeutics. Matt discusses the ongoing global problem of antimicrobial resistance (AMR). He suggests that lessons learned from the COVID-19 pandemic can inform both innovation in AMR therapies and our understanding of AMR. To learn more about the global challenge of AMR and brainstorm solutions, request an invitation to Innovate4Outcomes ®.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
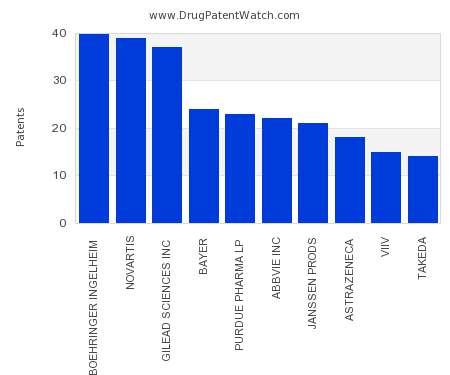
Drug Patent Watch
NOVEMBER 1, 2022
This chart shows the pharmaceutical companies with the most patents in Croatia. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is…. The post Which pharmaceutical companies have the most drug patents in Croatia? appeared first on DrugPatentWatch - Make Better Decisions.

DrugBank
NOVEMBER 3, 2022
This blog is part of a bigger series. Missed part one? Check out the blog post here or Download Your Guide to Quality Drug Data to get the whats, whys, and hows of quality drug data, according to our experts. Quality data will never be the result of a single metric or of executing on one dimension of quality perfectly. Rather, it is the sum of many crucial elements working together.

Speaker: Simran Kaur, Co-founder & CEO at Tattva.Health
AI is transforming clinical trials—accelerating drug discovery, optimizing patient recruitment, and improving data analysis. But its impact goes far beyond research. As AI-driven innovation reshapes the clinical trial process, it’s also influencing broader healthcare trends, from personalized medicine to patient outcomes. Join this new webinar featuring Simran Kaur for an insightful discussion on what all of this means for the future of healthcare!

Drug Discovery Today
NOVEMBER 3, 2022
Bowel cancer patients could in future benefit from a new 3D bioprinting technology which would use their own cells to replicate the complex cellular environment of solid tumours in 3D models. The University of Bristol-led advance, published in Biofabrication, would allow clinicians to treat the models, known as spheroids, with chemotherapy drugs and radiation to help them understand an individual patient’s resistance to therapies.
Drug Discovery Digest brings together the best content for drug research and development professionals from the widest variety of industry thought leaders.
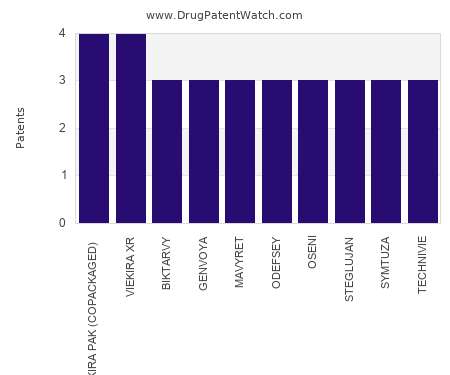
Drug Patent Watch
NOVEMBER 4, 2022
This chart shows the drugs with the most patents in Netherlands. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is desired.…. The post Which pharmaceutical drugs have the most drug patents in Netherlands? appeared first on DrugPatentWatch - Make Better Decisions.

Conversations in Drug Development Trends
NOVEMBER 3, 2022
BioPharma Dive interviewed our Derek Ansel on how to conduct psychedelic clinical research. In April 2022, the Food and Drug Administration (FDA) issued draft guidance on developing a “Race and Ethnicity Diversity Plan” for clinical trials. This plan, designed to help make clinical trial enrollment more representative, stands to support diversity, equity and inclusion (DEI) initiatives in R&D — a sector that certainly needs more attention across all clinical trials.

Drug Discovery Today
NOVEMBER 2, 2022
BOSTON, Mass. and Oxford, UK – November 2, 2022 – ROME Therapeutics and Enara Bio, two leading biotechnology companies focused on developing novel medicines driven by insights to the dark genome, today announced that they will host the inaugural Dark Genome Symposium on Monday, November 7, 2022 at ROME’s headquarters in Boston. This one-day event brings together leaders from academia, biotech and pharma working on the dark genome to share knowledge, opportunities, applications and challenges to

Antidote
NOVEMBER 2, 2022
We’re thrilled to share that today, we announced an important collaboration with Beyond Celiac, the leading celiac disease organization working to drive diagnosis, advance research, and accelerate the discovery of new treatments and a cure. We’ve been working with this incredible organization to make our celiac disease clinical trial search even better for patients and caregivers — and we’re now unveiling this blend of our matching technology with Beyond Celiac’s deep understanding of the condit

Speaker: Simran Kaur, Founder & CEO at Tattva Health Inc.
The healthcare landscape is being revolutionized by AI and cutting-edge digital technologies, reshaping how patients receive care and interact with providers. In this webinar led by Simran Kaur, we will explore how AI-driven solutions are enhancing patient communication, improving care quality, and empowering preventive and predictive medicine. You'll also learn how AI is streamlining healthcare processes, helping providers offer more efficient, personalized care and enabling faster, data-driven
Drug Patent Watch
NOVEMBER 4, 2022
Annual Drug Patent Expirations for SLYND Slynd is a drug marketed by Exeltis Usa Inc and is included in one NDA. There are nine patents protecting this drug. SLYND drug…. The post New patent for Exeltis Usa drug SLYND appeared first on DrugPatentWatch - Make Better Decisions.

PPD
NOVEMBER 1, 2022
Implementing functional service provider (FSP) partnerships is complex and requires significant resources and investments from each stakeholder in the early stages of a relationship. Partnerships involving a high volume of dedicated resources are complicated and time-intensive, requiring significant collaboration across multiple functions. To better manage these complexities, PPD created the implementation lead role to streamline the resource capacity management process and create efficiencies d

Reprocell
NOVEMBER 1, 2022
Pharma companies are legally required to test novel drugs in animal models before beginning human trials. And while animal testing has progressed thousands of therapeutics that we use today, it is not without its experimental and ethical downfalls. An article published in the Financial Times described how scientists are moving away from animal experimentation toward alternatives that more closely resemble human physiology. 1 The piece, titled "How science is getting closer to a world without ani

Antidote
OCTOBER 31, 2022
Clinical trial patient recruitment companies implement a range of methods, from digital advertising to community partnerships, to reach patients that might be a good fit for a study — but because there are many options for companies to work with, it is important that sites and sponsors thoroughly evaluate their options and choose the right vendor for their needs.

Advertisement
Clinical trial data management is increasingly challenging as studies grow in complexity. Quickly accessing and analyzing study data is vital for assessing trial progress and patient safety. In this paper, we explore real-time data access and analysis for proactive study management. We investigate using adverse event (AE) data to monitor safety and discuss a clinical analytics platform that supports collaboration and data review workflows.
Drug Patent Watch
NOVEMBER 4, 2022
Annual Drug Patent Expirations for KATERZIA Katerzia is a drug marketed by Azurity and is included in one NDA. It is available from one supplier. There are six patents protecting…. The post New patent for Azurity drug KATERZIA appeared first on DrugPatentWatch - Make Better Decisions.

ProRelix Research
OCTOBER 31, 2022
A clinical trial is a culmination of the several stages of a drug or medical device development program that begins with the discovery of a candidate molecule followed by preclinical […]. The post IND Data Requirements and US FDA Submission Process appeared first on ProRelix Research.

LifeSciVC
NOVEMBER 1, 2022
By Robert Clarke, CEO and founder of Kinaset Therapeutics, as part of the From The Trenches feature of LifeSciVC 2022 has been a hallmark year for Kinaset Therapeutics as we’ve continued to advance our P1b trial with our lead asset. We’ve also nearly doubled the size of the team…to 5 employees from 3. A testament to our team, we’ve received a fair amount of praise (and sometimes incredulity) from investors and business development colleagues alike with regard to the amount of progress we have ma

Drug & Device Law
NOVEMBER 3, 2022
This post is from the non-Reed Smith side of the blog. Defendants in Pizzitola v. Ethicon, Inc., filed motions to exclude two of plaintiff’s experts and both decisions (two orders issued) heavily favored the defense, rejecting recurrent design defect arguments by plaintiffs. The product at issue is synthetic pelvic mesh. Plaintiff’s first challenged expert was a gynecologic surgeon.

Advertisement
Clinical research has entered a new era, one that requires real-time analytics and visualization to allow trial leaders to work collaboratively and to develop, at the click of a mouse, deep insights that enable proactive study management. Learn how Revvity Signals helps drug developers deliver clinical trial data insights in real-time using a fast and flexible data and analytics platform to empower data-driven decision-making.
Drug Patent Watch
NOVEMBER 4, 2022
Annual Drug Patent Expirations for DROSPIRENONE Drospirenone is a drug marketed by Exeltis Usa Inc, Barr, Glenmark Pharms Ltd, Hetero Labs Ltd, Hlthcare, Jubilant Cadista, Mylan Labs Ltd, Watson Labs,…. The post New patent for Exeltis Usa drug DROSPIRENONE appeared first on DrugPatentWatch - Make Better Decisions.
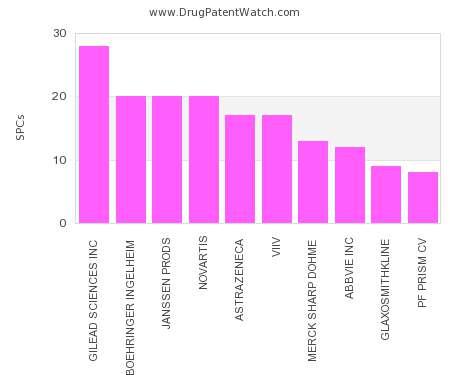
Drug Patent Watch
NOVEMBER 3, 2022
This chart shows the pharmaceutical companies with the most supplementary protection certificates (SPCs) in Lithuania. SPCs are used in European Union and select others to encourage pharmaceutical innovation by compensating…. The post Which pharmaceutical companies have the most SPCs in Lithuania? appeared first on DrugPatentWatch - Make Better Decisions.
Drug Patent Watch
NOVEMBER 3, 2022
Annual Drug Patent Expirations for TRINTELLIX Trintellix is a drug marketed by Takeda Pharms Usa and is included in one NDA. It is available from three suppliers. There are ten…. The post New patent for Takeda Pharms drug TRINTELLIX appeared first on DrugPatentWatch - Make Better Decisions.
Drug Patent Watch
NOVEMBER 3, 2022
Annual Drug Patent Expirations for YUPELRI Yupelri is a drug marketed by Mylan Ireland Ltd and is included in one NDA. It is available from one supplier. There are sixteen…. The post New patent for Mylan Ireland drug YUPELRI appeared first on DrugPatentWatch - Make Better Decisions.

Advertisement
Clinical development organizations face a wide array of challenges when it comes to data, many of which can impact the operational effectiveness of their clinical trials. In this whitepaper, experts from Revvity Signals explore how solutions like TIBCO® Spotfire® enable better, more streamlined studies. The whitepaper also features a success story from Ambrx, a leading biopharmaceutical company, detailing how it has leveraged Spotfire to tackle data quality and collaboration challenges in clinic
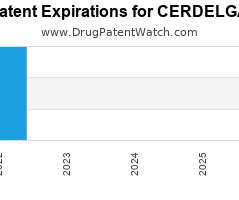
Drug Patent Watch
NOVEMBER 3, 2022
Annual Drug Patent Expirations for CERDELGA Cerdelga is a drug marketed by Genzyme Corp and is included in one NDA. It is available from one supplier. There are six patents…. The post New patent for Genzyme Corp drug CERDELGA appeared first on DrugPatentWatch - Make Better Decisions.

Drug Patent Watch
NOVEMBER 3, 2022
Annual Drug Patent Expirations for RECORLEV Recorlev is a drug marketed by Strongbridge and is included in one NDA. There are six patents protecting this drug. This drug has twenty-eight…. The post New patent for Strongbridge drug RECORLEV appeared first on DrugPatentWatch - Make Better Decisions.
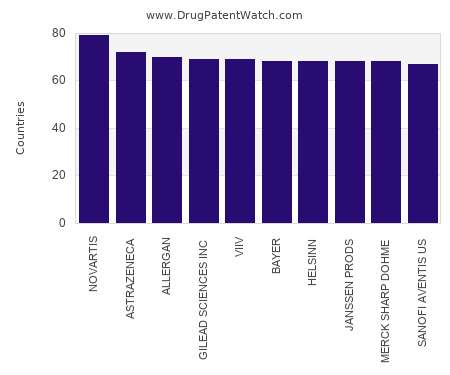
Drug Patent Watch
NOVEMBER 2, 2022
This chart shows the pharmaceutical companies with patents in the most countries. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is…. The post Which pharmaceutical companies have the greatest global patent coverage? appeared first on DrugPatentWatch - Make Better Decisions.
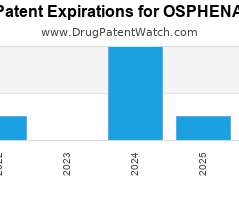
Drug Patent Watch
NOVEMBER 1, 2022
Annual Drug Patent Expirations for OSPHENA Osphena is a drug marketed by Duchesnay and is included in one NDA. It is available from one supplier. There are eight patents protecting…. The post New patent expiration for Duchesnay drug OSPHENA appeared first on DrugPatentWatch - Make Better Decisions.
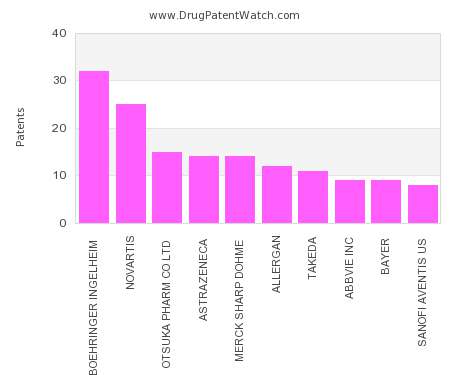
Drug Patent Watch
OCTOBER 31, 2022
This chart shows the pharmaceutical companies with the most patents in Malaysia. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is…. The post Which pharmaceutical companies have the most drug patents in Malaysia? appeared first on DrugPatentWatch - Make Better Decisions.
Drug Patent Watch
OCTOBER 31, 2022
Annual Drug Patent Expirations for OXBRYTA Oxbryta is a drug marketed by Global Blood Theraps and is included in two NDAs. There are nine patents protecting this drug. This drug…. The post New patent for Global Blood drug OXBRYTA appeared first on DrugPatentWatch - Make Better Decisions.
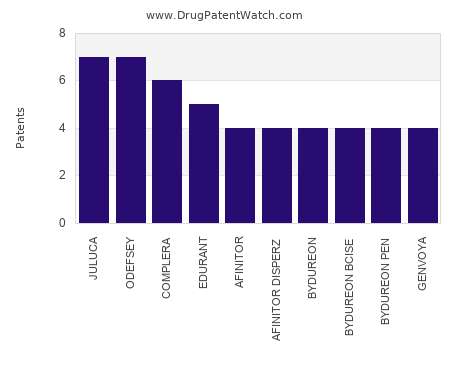
Drug Patent Watch
OCTOBER 29, 2022
This chart shows the drugs with the most patents in Luxembourg. Patents must be filed in each country (or, in some cases regional patent office) where patent protection is desired.…. The post Which pharmaceutical drugs have the most drug patents in Luxembourg? appeared first on DrugPatentWatch - Make Better Decisions.

Drug Patent Watch
NOVEMBER 4, 2022
[![saxagliptin structure]([link] Saxagliptin is the generic ingredient in one branded drug marketed by Astrazeneca Ab and is included in one NDA. There are two patents protecting this compound. There are…. The post New tentative approval for Amneal Pharms drug saxagliptin appeared first on DrugPatentWatch - Make Better Decisions.
Let's personalize your content