Sphere Fluidics closes a $40 million funding round led by Sofinnova Partners and Redmile Group
Drug Discovery Today
OCTOBER 28, 2021
Cambridge, UK, 28 October 2021: Sphere Fluidics, a company that has developed and is commercialising single cell analysis systems underpinned by its proprietary picodroplet technology, announced today that it has closed a $40 million (circa £30 million) investment round. The round was led by Sofinnova Partners (Paris, France) and Redmile Group (San Francisco, USA) co-investing on equal terms.
















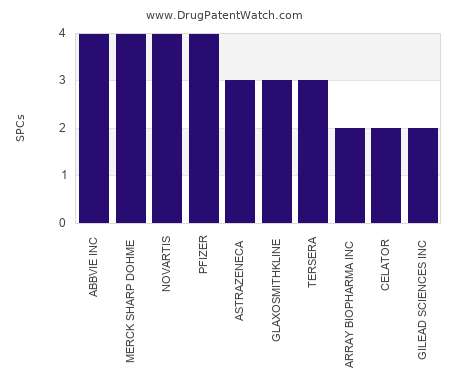




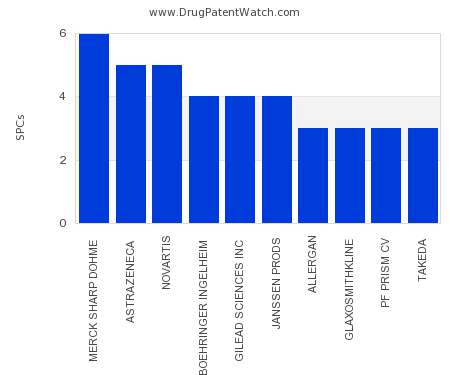






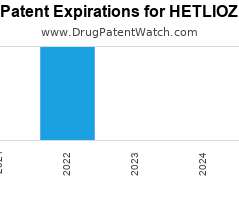








Let's personalize your content