Details of Adverse Event in AstraZeneca’s Vaccine Trial Still Unknown | 2020-10-15
The Pharma Data
OCTOBER 15, 2020
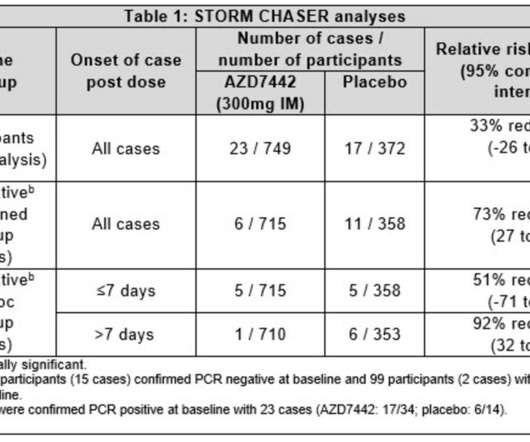
More than a month has passed since AstraZeneca’s phase 3 COVID-19 vaccine trial was paused due to a potential adverse event, but few solid details have emerged from ongoing safety reviews by the FDA and the UK drugmaker. The vaccine trial, which has resumed in other countries but remains halted in the U.S., James Miessler.














































Let's personalize your content