Evaluating the Abuse Potential of Lenabasum, a Selective CB2 Cannabinoid Receptor Agonist [Behavioral Pharmacology]
ASPET
JUNE 27, 2024
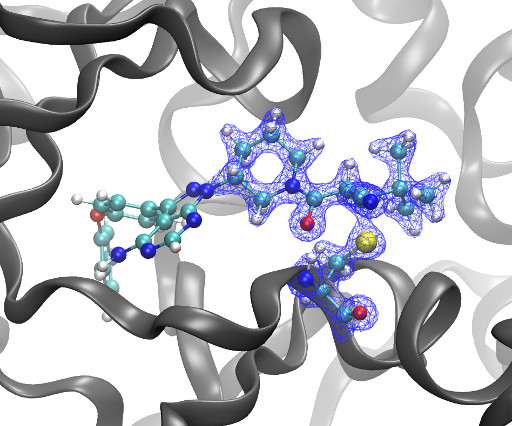
Secondary VAS and pharmacokinetic (PK) endpoints and adverse events were assessed. Three doses of lenabasum (20, 60, and 120mg) were compared to placebo, and nabilone (3 and 6mg). The primary endpoint was the peak effect (Emax) on a bipolar Drug Liking visual analog scale (VAS). Results : Lenabasum was safe and well tolerated.










































Let's personalize your content