ImmunityBio’s hAd5 T-Cell COVID-19 Vaccine Candidate Shows Complete Protection of Airways in Non-Human Primates
The Pharma Data
DECEMBER 10, 2020
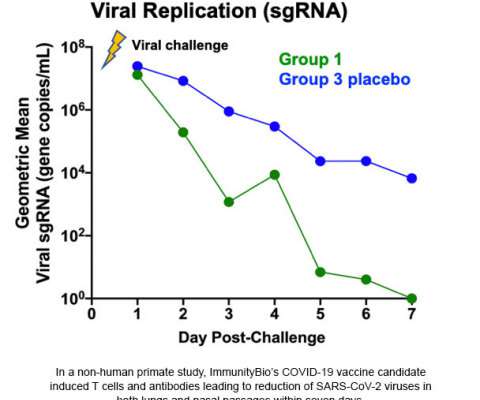
In the study, immunization with the hAd5-COVID-19 vaccine inhibited SARS-CoV-2 virus replication in 100% (10 of 10) of Rhesus macaques, with a drop in viral replication starting on the first day of vaccine administration, and undetectable viral levels as early as three to five days post-challenge in most of the animals.





















Let's personalize your content