Sanofi and GSK to seek regulatory authorization for COVID-19 vaccine
The Pharma Data
FEBRUARY 23, 2022
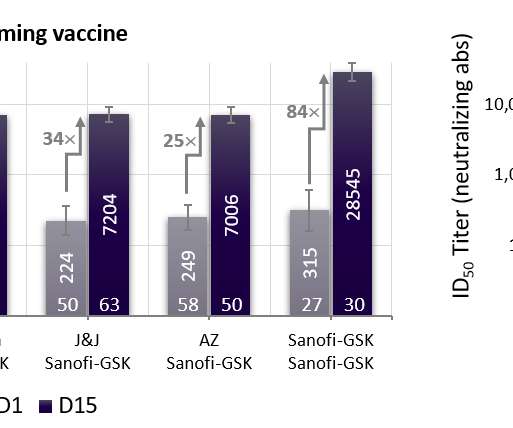
* Final analysis of the global VAT02 booster trial confirms universal ability to boost neutralizing antibodies 18- to 30-fold across vaccine platforms (mRNA, adenovirus). * efficacy against any symptomatic COVID-19 disease, in line with expected vaccine effectiveness in today’s environment dominated by variants of concern.





















Let's personalize your content