Insights into cellular therapies for cancer treatment
Drug Target Review
FEBRUARY 21, 2024
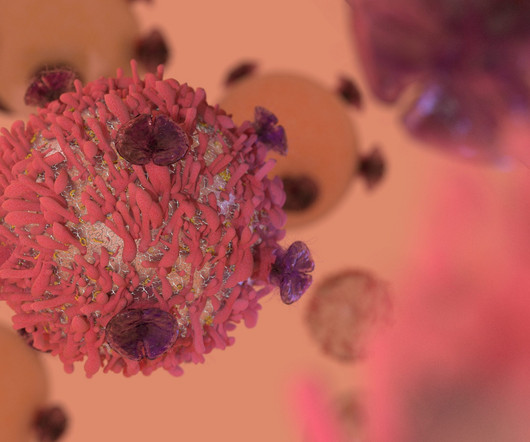
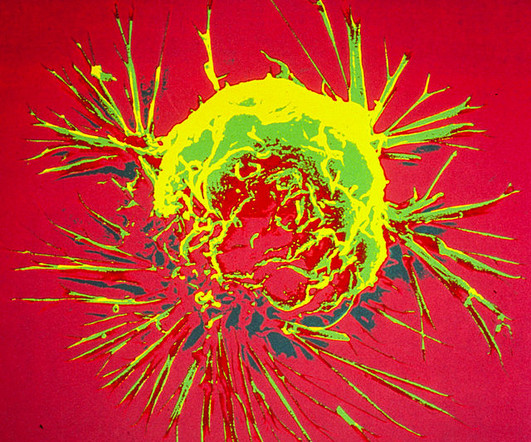
Stem cell transplants primarily help the immune response through the “graft-versus-leukaemia effect,” and we have to manage the “graft versus host effect.” This involves the use of CAR-T cell products derived from donor T cells, hence the term “off the shelf,” making it easier to standardise treatments.




































Let's personalize your content