New gene delivery vehicle shows promise for human brain gene therapy
Broad Institute
MAY 16, 2024
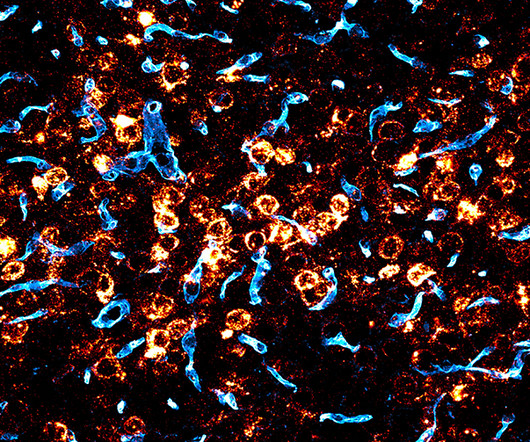
Gene therapy could potentially treat a range of severe genetic brain disorders, which currently have no cures and few treatment options. Since we came to the Broad we’ve been focused on the mission of enabling gene therapies for the central nervous system,” said Deverman, senior author on the study. “If










































Let's personalize your content