EC approves new combination treatment for NSCLC
Drug Discovery World
SEPTEMBER 2, 2024
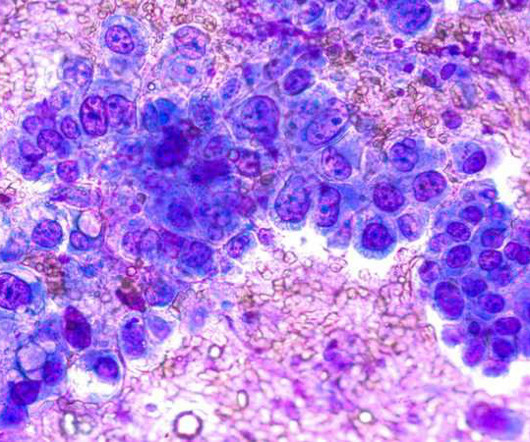
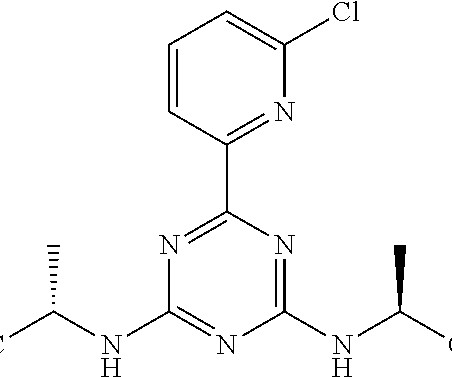
The European Commission (EC) has approved Braftovi (encorafenib) in combination with Mektovi (binimetinib) for the treatment of adult patients with advanced non-small cell lung cancer (NSCLC) with a BRAFV600E mutation.










































Let's personalize your content