Takeda secures FDA approval for colon cancer drug
BioPharma Drive: Drug Pricing
NOVEMBER 9, 2023
The pharma paid $400 million to license the drug from Hutchmed earlier this year in an effort to bolster its oncology business.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

BioPharma Drive: Drug Pricing
NOVEMBER 9, 2023
The pharma paid $400 million to license the drug from Hutchmed earlier this year in an effort to bolster its oncology business.
New Drug Approvals
APRIL 11, 2025
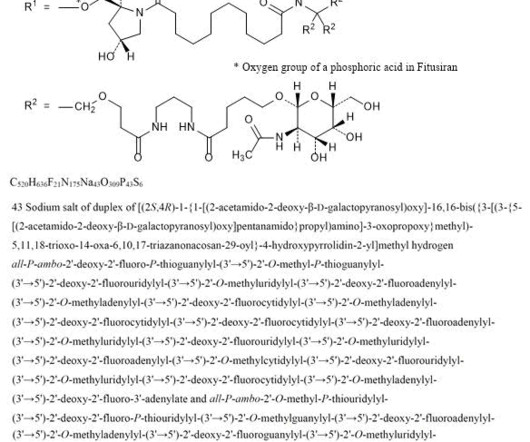
2] Fitusiran was approved for medical use in the United States in March 2025. [2] 2] The fixed dose of fitusiran is not approved because it led to excessive clotting in some participants. [2] 2] The US Food and Drug Administration (FDA) granted the application for fitusiran orphan drug and fast track designations. 26 March 2025.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

ASPET
OCTOBER 12, 2023
The Countering Emerging Threats - Rapid Acquisition and Investigation of Drugs for Repurposing (CET RAIDR) program within the JPM Medical is designed to rapidly tackle known, unknown, and emerging threats by utilizing late-stage or licensed therapeutics. Repurposing is one such method.

The Pharma Data
MAY 7, 2021
The Pfizer-BioNTech COVID-19 Vaccine has not been approved or licensed by the U.S. Food and Drug Administration (FDA), but has been authorized for emergency use by FDA under an Emergency Use Authorization (EUA) to prevent Coronavirus Disease 2019 (COVID-19) for use in individuals 16 years of age and older.

The Pharma Data
NOVEMBER 27, 2020
FDA Approves Danyelza (naxitamab-gqgk) for the Treatment of Neuroblastoma. It’s very exciting to see this treatment go from being an experimental therapy used at my daughter’s bedside to now being FDA approved. Related Articles: Danyelza (naxitamab-gqgk) FDA Approval History. NEW YORK, Nov. Source link.

The Pharma Data
DECEMBER 13, 2020
During the meeting, the FDA provided encouraging feedback regarding the Phase 3 study of omidubicel pertaining to the pre-specified primary and secondary endpoints. The FDA also recommended that Gamida Cell generate additional manufacturing-related data prior to requesting a pre-Biologics License Application (BLA) meeting.

FDA Law Blog: Drug Discovery
DECEMBER 7, 2022
Valentine — On November 22, 2022, FDA approved CSL Behring’s BLA for Hemgenix (etranacogene dezaparvovec), an AAV-based gene therapy for the treatment of adults with Hemophilia B who currently use Factor IX prophylaxis therapy, have current or historical life-threatening hemorrhage, or have repeated, serious spontaneous bleeding episodes.

The Pharma Data
OCTOBER 21, 2020
a biopharmaceutical company developing multiple assets in the ophthalmic and injectable areas, announced today it received approval from the U.S. Food and Drug Administration (FDA) to market Ephedrine Sulfate Injection in a ready-to-use 50mg/10 ml single use vial presentation. BRIDGEWATER, N.J., and Canada.
The Pharma Data
DECEMBER 9, 2020
.–( BUSINESS WIRE )– Advanced Bionics (AB) , a global leader in cochlear implant technology, in collaboration with Phonak, a leading provider of life-changing hearing solutions, receives FDA approval and announces it is bringing Marvel hearing technology to Advanced Bionics cochlear implant wearers. Life is on. Gifford, R.

The Pharma Data
DECEMBER 16, 2020
CNS holds a worldwide exclusive license to the Berubicin chemical compound and has acquired all data and know-how from Reata Pharmaceuticals, Inc. Its lead drug candidate, Berubicin, is proposed for the treatment of glioblastoma multiforme (GBM), an aggressive and incurable form of brain cancer.

The Pharma Data
AUGUST 21, 2020
The FDA has approved Genmab and Janssen’s Darzalex (daratumumab) as a treatment for relapsed and refractory multiple myeloma, when combined with carfilzomib and dexamethasone, in patients who have previously received between one and three lines of therapy. Multiple myeloma affects 26,000 new patients in America every year.

The Pharma Data
NOVEMBER 30, 2020
1, 2020 /PRNewswire/ — Sosei Group Corporation (“the Company”) (TSE: 4565) announces it has entered into a global collaboration and license agreement with Biohaven Pharmaceutical Holding Company Ltd. (“Biohaven”, NYSE: BHVN). . TOKYO and CAMBRIDGE, England , Dec.
New Drug Approvals
SEPTEMBER 8, 2024
1] [2] [3] The compound was licensed from Janssen Pharmaceutica NV. [4] 4] Seladelpar was approved for medical use in the United States in August 2024. [1] 1] [2] [3] The compound was licensed from Janssen Pharmaceutica NV. [4] 4] Seladelpar was approved for medical use in the United States in August 2024. [1]

New Drug Approvals
DECEMBER 19, 2022
FDA 12/1/2022, To treat adults with relapsed or refractory acute myeloid leukemia with a susceptible isocitrate dehydrogenase-1 (IDH1) mutation, Rezlidhia Olutasidenib , sold under the brand name Rezlidhia , is an anticancer medication used to treat relapsed or refractory acute myeloid leukemia. [1] 1] It is taken by mouth. [1] Hz, 1 H), 4.62−4.75
New Drug Approvals
APRIL 2, 2025
1] [2] It was developed by Vertex Pharmaceuticals , [5] and was approved for medical use in the United States in January 2025. [2] 2] [6] Suzetrigine is the first medication to be approved by the US Food and Drug Administration (FDA) in this new class of pain management medicines. [2] Food and Drug Administration (FDA).

The Pharma Data
APRIL 14, 2021
Food and Drug Administration (FDA) has approved the company’s supplemental Biologics License Application for Xolair® (omalizumab) prefilled syringe for self-injection across all approved U.S. with Xolair since its initial approval in 2003. today announced that the U.S. indications.

The Pharma Data
MAY 4, 2021
Pfizer plans to file for full FDA approval of Covid vaccine at the end of this month ( CNBC ). The FDA is set to authorize the Pfizer-BioNTech vaccine for those 12-15 years old by early next week. FDA approves expanded use for Chiesi’s sickle cell drug Ferriprox ( PMLive ).

The Pharma Data
JANUARY 18, 2021
In total, there were 31 mostly virtual expert panel meetings in 2020, but more than a dozen of those did not involve votes on New Drug Applications (NDAs), Biologics License Applications (BLAs) or new indications, but instead focused on devices, tobacco or other topics.
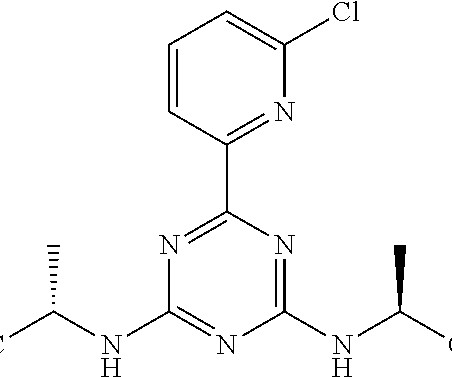
New Drug Approvals
SEPTEMBER 10, 2024
2] Vorasidenib was approved for medical use in the United States in August 2024. [2] 2] [3] It is the first approval by the US Food and Drug Administration (FDA) of a systemic therapy for people with grade 2 astrocytoma or oligodendroglioma with a susceptible isocitrate dehydrogenase-1 or isocitrate dehydrogenase-2 mutation. [2]

Policy Prescription
OCTOBER 3, 2023
That’s why, according to Salant, drug companies “[blur] the distinction between dangerous counterfeit drugs and safe drugs sold by pharmacies licensed in other high-income countries.” Of course, India is the largest supplier of FDA-approved generic drugs sold in U.S. Many people in the U.S.,

New Drug Approvals
SEPTEMBER 6, 2024
2 , 3 Lazertinib was first approved in South Korea on January 18, 2021, for the treatment of EGFR T790M mutation-positive non-small cell lung cancer (NSCLC) with EGFR mutations. 1 It was approved by the FDA on August 19, 2024. 1 It was approved by the FDA on August 19, 2024. Food and Drug Administration (FDA).

The Pharma Data
APRIL 4, 2022
If approved, Actemra/RoActemra would be the first U.S. FDA approval is expected in the second half of this year. Food and Drug Administration (FDA)-approved for this use and there is limited information known about the safety or effectiveness of using Actemra/RoActemra to treat people in the hospital with COVID-19.

FDA Law Blog: Biosimilars
AUGUST 8, 2024
Rescheduling out of schedule I would allow for the medical use of FDA-approved prescription drugs dispensed by DEA-registered, state licensed pharmacies pursuant to prescriptions issued by similarly DEA-registered, state licensed practitioners.

New Drug Approvals
SEPTEMBER 20, 2023
1] Motixafortide was approved for medical use in the United States in September 2023. [2] 4 Similar in mechanism to the previously approved plerixafor , motixafortide is an inhibitor of C-X-C Motif Chemokine Receptor 4 (CXCR4), a protein that helps to anchor stem cells to bone marrow matrix. 1] It is given by subcutaneous injection. [1]

New Drug Approvals
DECEMBER 24, 2023
S2CID 250989659. ^ “Eplontersen: FDA-Approved Drugs” U.S. Food and Drug Administration (FDA). Retrieved 21 December 2023. ^ “Wainua (eplontersen) granted regulatory approval in the U.S. 88 (12): 5389–5398. doi : 10.1111/bcp.15468. PMID 35869634.

New Drug Approvals
DECEMBER 24, 2023
3] In November 2023, capivasertib was approved in the United States for people with hormone receptor-positive, human epidermal growth factor receptor 2 -negative breast cancer when used in combination with fulvestrant. [3] Jump up to: a b c d e f “FDA approves capivasertib with fulvestrant for breast cancer” U.S.

FDA Law Blog: Biosimilars
JULY 5, 2023
As a reminder, in Title I of the 2013 Drug Quality and Security Act (DQSA) (the Compounding Quality Act), Congress created the “outsourcing facility” FDA registration category, and set forth statutory parameters for their operation in new section 503B of the FDCA. See 21 U.S.C. 353b(a). Section II at 2. Draft Guidance III.B.2(e)
The Pharma Data
AUGUST 16, 2021
Phase 3 results evaluating the third dose are expected shortly and will be submitted to the FDA, the EMA and other regulatory authorities worldwide. Submissions to pursue regulatory approvals in those countries where emergency use authorizations or equivalent were initially granted are ongoing or planned. In the U.S.,

The Pharma Data
NOVEMBER 29, 2020
Y-mAbs Therapeutics has a target action date of November 30 for its Biologics License Application (BLA) for Danyelza (naxitamab) for patients with relapsed/refractory high-risk neuroblastoma. The drug was developed by researchers at Memorial Sloan Kettering Cancer Center and exclusively licensed to Y-mAbs.

New Drug Approvals
OCTOBER 24, 2023
Molecular Weight: 631.700 FDA APPROVED, To treat moderately to severely active ulcerative colitis in adults, 10/12/2023 Velsipity Etrasimod , sold under the brand name Velsipity , is a medication that is used for the treatment of ulcerative colitis (UC). [1] “FDA Approves New Drug for Ulcerative Colitis” Medscape.
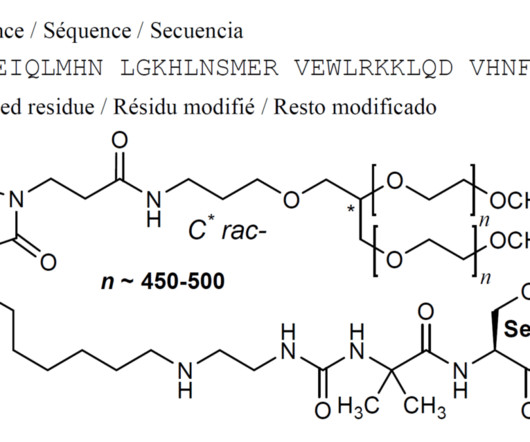
New Drug Approvals
SEPTEMBER 8, 2024
Jump up to: a b c d e f g h “FDA approves new drug for hypoparathyroidism, a rare disorder” U.S. Food and Drug Administration (FDA) (Press release). Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged. 9 August 2024.

The Pharma Data
AUGUST 19, 2021
Food and Drug Administration (FDA) approval for patients with locally advanced or metastatic NSCLC with epidermal growth factor receptor (EGFR) exon 20 insertion mutations, based on data showing an ORR of 40 percent (95 percent CI, 29 – 51) and median duration of response of 11.1 months (95 percent CI, 6.9 – NE). [7].

The Pharma Data
JANUARY 5, 2021
based contract manufacturing business, Benuvia Manufacturing, which has significant chemistry and formulation capabilities, including manufacturing our FDA-approved cannabinoid drug, SYNDROS ® ,” said Todd C. We look forward to supporting Radius through our U.S. Davis, executive chairman of Benuvia. Disease Highlights.

New Drug Approvals
DECEMBER 16, 2023
Retrieved 10 December 2023. ^ “Novartis receives FDA approval for Fabhalta (iptacopan), offering superior hemoglobin improvement in the absence of transfusions as the first oral monotherapy for adults with PNH” Novartis (Press release). 5 December 2023. . twitter +919321316780 call whatsaapp EMAIL. 5 December 2023.

The Pharma Data
JANUARY 13, 2021
Brilacidin for UP/UPS was licensed to Alfasigma S.p.A. A Phase 2b trial of Brilacidin showed a single intravenous dose of the drug delivered comparable outcomes to a seven-day dosing regimen of the FDA-approved blockbuster daptomycin in treating Acute Bacterial Skin and Skin Structure Infection.

FDA Law Blog: Biosimilars
FEBRUARY 26, 2024
As FDA explains, Boehringer’s request would allow its “Cyltezo (adalimumab-adbm) injection, which contains the same total content of drug substance and same concentration as Original Concentration Humira (e.g., mL), to be biosimilar to or interchangeable with High Concentration Humira (e.g., mL) in addition to Original Concentration Humira.”

The Pharma Data
JUNE 28, 2021
The Breakthrough Therapy designation aims to expedite the development and review of drugs that are intended to treat a serious condition when preliminary clinical evidence indicates that the drug may demonstrate substantial improvement on a clinically significant endpoint(s) over already available therapies that have received full FDA approval.

The Pharma Data
AUGUST 19, 2021
The application will be reviewed by the EMA’s Committee for Medicinal Products for Human Use (CHMP) under the centralized licensing procedure for all 27 Member States of the European Union, as well as Norway, Iceland and Liechtenstein. Additional data from the CAPELLA study will be presented at a future scientific conference.

FDA Law Blog: Biosimilars
FEBRUARY 29, 2024
The use of a surrogate endpoint can considerably shorten the time required prior to receiving FDA approval. If the confirmatory trial shows that the drug actually provides a clinical benefit, then the FDA grants traditional approval for the drug.

The Pharma Data
OCTOBER 27, 2021
Filings supported by pivotal ELARA trial, where treatment with Kymriah showed robust response rates and remarkable safety profile in adult patients with relapsed or refractory (r/r) follicular lymphoma (FL) 1 Kymriah also received orphan drug designation from the European Commission (EC) for patients with FL earlier this year Patients with FL who are (..)

The Pharma Data
MARCH 4, 2021
Food and Drug Administration (FDA) has accepted for review the supplemental Biologics License Application (sBLA) for Dupixent ® (dupilumab) as an add-on treatment for children aged 6 to 11 years with uncontrolled moderate-to-severe asthma.

The Pharma Data
NOVEMBER 2, 2020
Currently, Amazon only offers special license for point of care and professional use medical products, which would preclude the individual use GenViro kit from being available on Amazon, even after FDA approval is secured. Two of the company’s.

The Pharma Data
JUNE 7, 2022
. “We look forward to providing an important new medicine and helping patients find the relief they so desperately seek from the varied and debilitating symptoms of this disease, contingent upon FDA approval.” With these data, Lilly plans to submit a Biologics License Application (BLA) to the U.S. Almirall S.A.’s

The Pharma Data
MAY 2, 2022
Under the terms of the settlement agreement, the litigation between the parties in the United States District Court for the District of New Jersey will be ended, and Lupin will have a license to sell its generic product beginning April 2033, or earlier under certain circumstances.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content