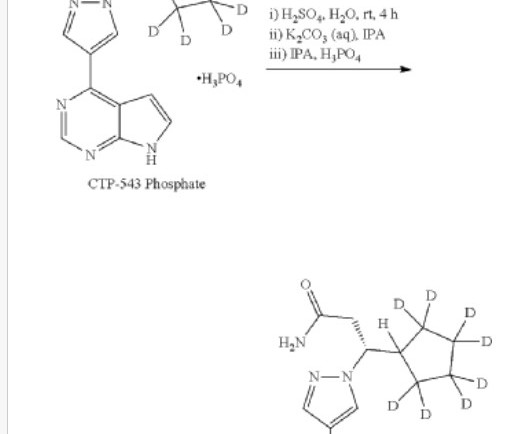
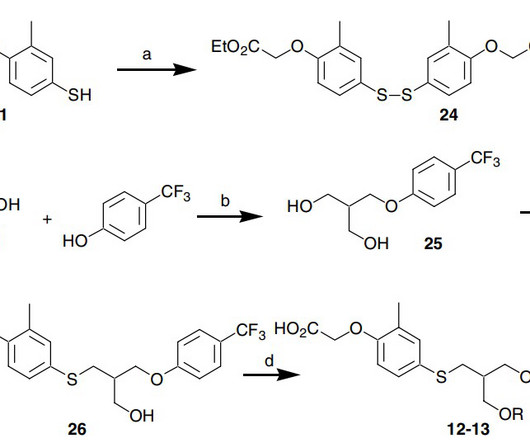
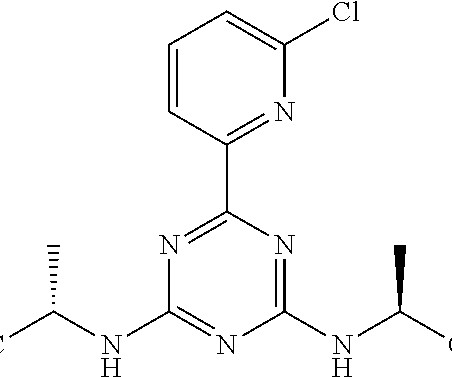
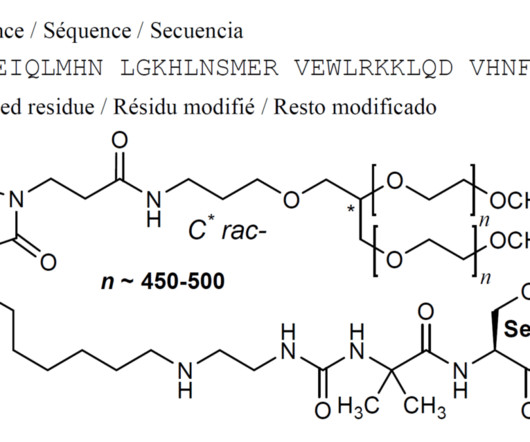
Hanmi Pharmaceutical expects U.S. FDA approval for 2 new drugs
The Pharma Data
JANUARY 17, 2021
18 , 2021 /PRNewswire/ — Hanmi Pharmaceutical Co., In particular, two new drugs developed by Hanmi Pharmaceutical Co., are expected to be approved by the U.S. . In particular, two new drugs developed by Hanmi Pharmaceutical Co., are expected to be approved by the U.S.












































Let's personalize your content