Pfizer wins FDA approval for its $7B colitis drug
BioPharma Drive: Drug Pricing
OCTOBER 13, 2023
Acquired with the buyout of Arena Pharmaceuticals, Velsipity enters a competitive market for ulcerative colitis pills.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

BioPharma Drive: Drug Pricing
OCTOBER 13, 2023
Acquired with the buyout of Arena Pharmaceuticals, Velsipity enters a competitive market for ulcerative colitis pills.
Drugs.com
NOVEMBER 17, 2023
16, 2023 -- The first home test for chlamydia and gonorrhea will soon hit the market, following its approval Wednesday by the U.S. THURSDAY, Nov. Food and Drug Administration. People will be able to buy the Simple 2 Test over-the-counter at a.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

BioPharma Drive: Drug Pricing
AUGUST 29, 2023
A broadened clearance for Reblozyl in myelodysplastic syndromes should help Bristol Myers offset the looming loss of revenue from top-selling medicines set to soon lose market exclusivity.

BioPharma Drive: Drug Pricing
AUGUST 25, 2023
According to one analyst, the approval was the last hurdle keeping Sandoz’s Tyruko from directly competing in the U.S. market against Biogen’s inflammation-regulating medicine Tysabri.

Drugs.com
JANUARY 17, 2025
Food and Drug Administration has authorized the marketing of 20 ZYN nicotine pouch products. FRIDAY, Jan. 17, 2025 -- Following an extensive scientific review, the U.S. Nicotine pouches -- small synthetic fiber pouches containing nicotine -- are.

Drugs.com
APRIL 1, 2025
Food and Drug Administration granted marketing authorization for the first home-based, nonprescription diagnostic test for chlamydia, gonorrhea, and trichomoniasis in women, the agency announced Friday.Women with. TUESDAY, April 1, 2025 -- The U.S.

BioPharma Drive: Drug Pricing
AUGUST 5, 2023
The agency turned back the companies' attempt to also win clearance for major depressive disorder, limiting its market potential.

Drugs.com
JUNE 13, 2023
Food and Drug Administration has approved over-the-counter marketing for the product, called Eroxon, as a. TUESDAY, June 13, 2023 -- Men with erectile dysfunction will now have the option of using a topical gel to treat the condition.

Drugs.com
JUNE 13, 2023
Food and Drug Administration has approved over-the-counter marketing for the product, called Eroxon, as a. TUESDAY, June 13, 2023 -- Men with erectile dysfunction will now have the option of using a topical gel to treat the condition.

BioPharma Drive: Drug Pricing
JUNE 16, 2023
The approval of Columvi adds another “bispecific antibody” to the market, highlighting the fast research progress for drugs that target two proteins rather than one.

BioPharma Drive: Drug Pricing
FEBRUARY 26, 2024
The companies didn’t reveal their planned price for Simlandi, which will be the 10th Humira copycat to reach the U.S.

Drugs.com
JUNE 13, 2023
Food and Drug Administration has approved over-the-counter marketing for the product, called Eroxon, as a. TUESDAY, June 13, 2023 -- Men with erectile dysfunction will now have the option of using a topical gel to treat the condition.

SCIENMAG: Medicine & Health
JULY 26, 2023
July 26, 2023 – Octapharma USA today announced that Balfaxar® (prothrombin complex concentrate, human-lans; marketed in Europe and Canada as octaplex®) has received U.S. PARAMUS, N.J.,

The Pharma Data
JANUARY 21, 2021
Cabotegravir is a ViiV product marketed as Cabenuva, and rilpivirine is a Janssen product with a brand name Edurant. Today’s FDA approval of Cabenuva represents a shift in the way HIV is treated, offering people living with HIV a completely new approach to care,” said Lynn Baxter, Head of North America, ViiV Healthcare. “Not

The Pharma Data
NOVEMBER 27, 2020
FDA Approves Danyelza (naxitamab-gqgk) for the Treatment of Neuroblastoma. It’s very exciting to see this treatment go from being an experimental therapy used at my daughter’s bedside to now being FDA approved. NEW YORK, Nov. 25, 2020 (GLOBE NEWSWIRE) — Y-mAbs Therapeutics, Inc.

The Pharma Data
DECEMBER 15, 2020
FDA Approves Klisyri (tirbanibulin) for the Treatment of Actinic Keratosis on the Face or Scalp. Klisyri is the first FDA approved branded proprietary product for Athenex and will be launched in partnership with Almirall in the U.S. The FDA approval of Klisyri is a significant milestone for Athenex. “The

The Pharma Data
NOVEMBER 2, 2020
FDA Approves Bronchitol (mannitol) Inhalation Powder to Improve Pulmonary Function in Adult Patients with Cystic Fibrosis. Food and Drug Administration (FDA) approval of Bronchitol (mannitol) inhalation powder. Food and Drug Administration (FDA) approval of Bronchitol (mannitol) inhalation powder.

The Pharma Data
AUGUST 31, 2021
10), as determined by an FDA-approved test, or in patients who were not eligible for any platinum-containing chemotherapy regardless of PD-L1 status. This indication was approved under accelerated approval based on tumor response rate and duration of response.
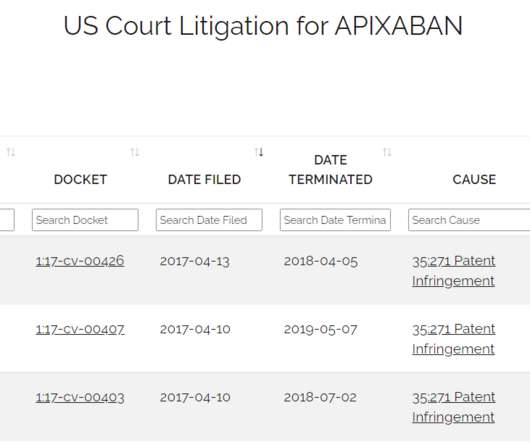
Drug Patent Watch
AUGUST 12, 2020
Just because a drug has received FDA approval does not mean that it is available in the marketplace. The post Generic Drugs Approved but not Launched – How to Tell When Generic Drugs Will hit the Market appeared first on DrugPatentWatch - Make Better Decisions.

The Pharma Data
NOVEMBER 27, 2020
FDA Approves Imcivree (setmelanotide) for Chronic Weight Management in Patients with Obesity Due to POMC, PCSK1 or LEPR Deficiency. With this approval, Imcivree becomes the first-ever FDA approved therapy for these rare genetic diseases of obesity. BOSTON, Nov. in the first quarter of 2021.

The Pharma Data
JANUARY 11, 2021
based subsidiary of Terumo and a global neurovascular company, announced today the FDA Approval of the PMA Supplement for the WEB 17 System, a new addition to the WEB Aneurysm Embolization System for the treatment of intracranial wide neck bifurcation aneurysms. . ALISO VIEJO, Calif. ,

SCIENMAG: Medicine & Health
NOVEMBER 16, 2023
Over the last four decades, insulin manufacturers have extended their periods of market exclusivity on brand-name insulin products by employing several strategies, including filing additional patents on their products after FDA approval and obtaining many patents on delivery devices for their insulin products.

The Pharma Data
OCTOBER 30, 2020
Dive into this week’s update for more details on the actions taken by the FDA in the ongoing response to the Covid-19 pandemic. FDA approves first treatment for Covid-19. On October 22, the FDA approved the antiviral drug Veklury for use in adult and pediatric patients for the treatment of Covid-19 requiring hospitalization.

The Pharma Data
MAY 19, 2023
ABBOTT RECEIVES FDA APPROVAL FOR TACTIFLEX™ ABLATION CATHETER FOR TREATMENT OF ABNORMAL HEART RHYTHM Abbott (NYSE: ABT) today announced that the U.S. Unlike other catheters on the market, the TactiFlex catheter uses a tip design with a laser-cut pattern that flexes when in contact with the heart wall.

The Pharma Data
JUNE 28, 2023
FDA Approves Pfizer’s NGENLA™, a Long-Acting Once-Weekly Treatment for Pediatric Growth Hormone Deficiency NEW YORK & MIAMI–(BUSINESS WIRE)– Pfizer Inc. NGENLA is approved for the treatment of pediatric GHD in more than 40 markets including Canada, Australia, Japan, and EU Member States.

The Pharma Data
OCTOBER 31, 2020
.” According to Global Data, the global market for influenza antivirals reached 2.34 Currently the market is comprised primarily of the neuraminidase inhibitor oseltamivir and the newly developed endonuclease inhibitor baloxavir. billion USD in 2019 and is estimated to reach 5.03 billion USD by 2026 at a CAGR of 11.5%.

ASPET
OCTOBER 12, 2023
The CET RAIDR program conserves both time-to-market and funds by leveraging previous conventional development work as a launch point for repurposing efforts. The focus of the CET RAIDR effort is to bridge treatment gaps between threat identification and the implementation of licensed targeted MCMs, thereby strengthening warfighter resiliency.
The Pharma Data
NOVEMBER 2, 2020
If nabiximols is approved, this would mark the second cannabis-based product for GW Pharmaceuticals in the United States. In 2018, the FDA approved Epidiolex as a treatment for seizures associated with two rare forms of epilepsy, Lennox-Gastaut syndrome (LGS) and Dravet syndrome. It also forced the U.S.

The Pharma Data
OCTOBER 21, 2020
a biopharmaceutical company developing multiple assets in the ophthalmic and injectable areas, announced today it received approval from the U.S. Food and Drug Administration (FDA) to market Ephedrine Sulfate Injection in a ready-to-use 50mg/10 ml single use vial presentation. BRIDGEWATER, N.J., and Canada.
The Pharma Data
JULY 29, 2021
Biosimilars marketed in the U.S. The substitution may occur at the pharmacy, a practice commonly called “pharmacy-level substitution” – much like how generic drugs are substituted for brand name drugs, subject to state pharmacy laws, which vary by state. More than 34 million people in the U.S.
The Pharma Data
AUGUST 20, 2020
Our commitment to the MS community stems all the way back to our initial investment in 2009 to bring a first generic Copaxone to market, which we achieved in 2017,” commented Mylan President Rajiv Malik. Mylan was quick to celebrate the decision and what it means for MS patients, though no information on the price of the drug was forthcoming.

The Pharma Data
AUGUST 19, 2021
today announced that the company’s Marketing Authorization Application (MAA) for lenacapavir, an investigational, long-acting HIV-1 capsid inhibitor, has been fully validated and is now under evaluation with the European Medicines Agency (EMA). Additional data from the CAPELLA study will be presented at a future scientific conference.

Advarra
JULY 26, 2022
Even though CBD and cannabis usage has grown, and marketing claims have proliferated regarding potential therapeutic applications, there is still little research on their effects on the human body. Cannabis and cannabis-derived compounds are treated the same as other FDA-regulated products and subject to the same authorities and requirements.
The Pharma Data
DECEMBER 3, 2020
The European Medicines Agency (EMA) is also reviewing the marketing authorization application (MAA) for the drug under the centralized procedure. An opinion from the Committee for Medicinal Products for Human Use is predicted to arrive within 12 months from the validation of the MAA, which was announced back on March 30 of this year.

Fierce BioTech
OCTOBER 5, 2023
Discover Strategies for Combatting Disruptions in Gene Therapy Development Cell and gene therapy development has exploded, with Q4 2022 showing more FDA approvals than over the past five years combined.[1] 1] How can sponsors keep pace with this rapidly growing market and avoid costly delays that can threaten critical timelines?

The Pharma Data
JULY 8, 2021
With a global network of R&D facilities, manufacturing sites and marketing subsidiaries, we strive to realize our hhc philosophy by delivering innovative products to target diseases with high unmet medical needs, with a particular focus in our strategic areas of Neurology and Oncology.

DS in Pharmatics
OCTOBER 14, 2022
An Investigational Device Exemption (IDE) is an application submitted to obtain the FDA's approval for use of a novel medical device in a clinical study. This allows for the collection of safety and effectiveness data in order to support full market approval. IDE applications support several types of studies: to […]

Agency IQ
SEPTEMBER 15, 2023
Until recently, the FDA relied on a monograph process through which firms could bring OTC drugs to market without FDA approval so long as it adhered to pre-set terms under the monograph. Fill out the form to read the full article.

The Pharma Data
MAY 7, 2021
BioNTech is the Marketing Authorization Holder in the European Union, and the holder of emergency use authorizations or equivalent in the United States (together with Pfizer), United Kingdom, Canada and other countries in advance of a planned application for full marketing authorizations in these countries.
Broad Institute
JULY 17, 2024
Drug toxicity can be an issue even after FDA approval; drug-induced cardiotoxicity (DICT) and drug-induced liver injury (DILI) each contribute to a significant percentage of post-market drug withdrawals. Seal began this work after wondering if more toxicology insights could be gleaned from a drug candidate’s chemical structure.

FDA Law Blog: Biosimilars
JUNE 27, 2023
Amongst his accomplishments, Law360 considered the role James has played in leveraging little-used pathways to FDA approval for often first-ever drugs to treat rare diseases (e.g., James was only one of five life sciences attorneys selected and the only food and drug lawyer to make the list.

Drug Target Review
MAY 15, 2024
3 The value required is more than just a greater number of FDA approvals, it is also about completing milestones with limited capital – ie, biotech survival. The latter group (ie, most of us) are waiting for wet lab validation, market demonstration (FDA approvals), and a few other things (which we often cannot define).

Fierce BioTech
SEPTEMBER 11, 2023
Aligning cross-functional teams across the organization to ensure medical, marketing, and market access are all in lock-step is even more crucial when launching a therapy into a rare disease market.

Policy Prescription
OCTOBER 3, 2023
Salant’s paper supports personal drug importation and legalizing the importation of prescription drugs for commercial use so that wholesale pharmacies, including companies like Amazon and Costco, could tap into the parallel importation markets of other countries. drug market is connected to foreign markets with lower drug prices.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content