Pfizer wins FDA approval for its $7B colitis drug
BioPharma Drive: Drug Pricing
OCTOBER 13, 2023
Acquired with the buyout of Arena Pharmaceuticals, Velsipity enters a competitive market for ulcerative colitis pills.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

BioPharma Drive: Drug Pricing
OCTOBER 13, 2023
Acquired with the buyout of Arena Pharmaceuticals, Velsipity enters a competitive market for ulcerative colitis pills.

The Pharma Data
NOVEMBER 2, 2020
FDA Approves Bronchitol (mannitol) Inhalation Powder to Improve Pulmonary Function in Adult Patients with Cystic Fibrosis. Food and Drug Administration (FDA) approval of Bronchitol (mannitol) inhalation powder. Food and Drug Administration (FDA) approval of Bronchitol (mannitol) inhalation powder.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

The Pharma Data
NOVEMBER 27, 2020
FDA Approves Danyelza (naxitamab-gqgk) for the Treatment of Neuroblastoma. It’s very exciting to see this treatment go from being an experimental therapy used at my daughter’s bedside to now being FDA approved. NEW YORK, Nov. 25, 2020 (GLOBE NEWSWIRE) — Y-mAbs Therapeutics, Inc.

The Pharma Data
DECEMBER 15, 2020
FDA Approves Klisyri (tirbanibulin) for the Treatment of Actinic Keratosis on the Face or Scalp. Klisyri is the first FDA approved branded proprietary product for Athenex and will be launched in partnership with Almirall in the U.S. The FDA approval of Klisyri is a significant milestone for Athenex. “The

The Pharma Data
NOVEMBER 27, 2020
FDA Approves Imcivree (setmelanotide) for Chronic Weight Management in Patients with Obesity Due to POMC, PCSK1 or LEPR Deficiency. 27, 2020 (GLOBE NEWSWIRE) — Rhythm Pharmaceuticals, Inc. With this approval, Imcivree becomes the first-ever FDA approved therapy for these rare genetic diseases of obesity.

Pharmaceutical Development Group
AUGUST 9, 2021
Formulation chemistry is the systematic and step-by-step approach to pharmaceutical development. Nowadays, as the usage of medicines is increasing, pharmaceutical companies are more eager to bring manufacturing a new look in quality and performance. There are different tests to assess the quality of a pharmaceutical product.

Advarra
JULY 27, 2022
As a pharmaceutical product makes its way through the lifecycle, there are often Food and Drug Administration (FDA) guidelines organizations should pay particular attention to. Go-to-market Strategy. If your go-to-market strategy requires FDA approval, it may also require a prior approval supplement.
The Pharma Data
NOVEMBER 2, 2020
GW Pharmaceuticals hopes to bring its cannabis-based treatment for multiple sclerosis spasticity to the United States. Sativex is approved for use in parts of Europe for this indication. Justin Gover, chief executive officer of GW Pharmaceuticals, proclaimed his excitement about launching the Phase III study in the United States.

The Pharma Data
OCTOBER 21, 2020
a biopharmaceutical company developing multiple assets in the ophthalmic and injectable areas, announced today it received approval from the U.S. Food and Drug Administration (FDA) to market Ephedrine Sulfate Injection in a ready-to-use 50mg/10 ml single use vial presentation. BRIDGEWATER, N.J., and Canada.

New Drug Approvals
APRIL 18, 2025
2] Crinecerfont was approved for medical use in the United States in December 2024. [2] 2] [3] The US Food and Drug Administration (FDA) considers it to be a first-in-class medication. [4] 2] The FDA granted the approval of Crenessity to Neurocrine Biosciences, Inc. [2] Food and Drug Administration (FDA) (Press release).
The Pharma Data
JULY 29, 2021
Biosimilars marketed in the U.S. The FDA released new materials for health care providers to enhance understanding about biosimilar and interchangeable biosimilar products, including a fact sheet about interchangeable biosimilar products. The FDA granted approval of Semglee (insulin glargine-yfgn) to Mylan Pharmaceuticals Inc.

DrugBank
DECEMBER 18, 2024
A new shift is occurring in the pharmaceutical industry, leading to a rapidly expanding field known as digital therapeutics (DTx). DTx interventions include sensor-equipped wearable devices, remote patient monitoring tools, and virtual reality platforms integrated with conventional pharmaceutical treatments.

FDA Law Blog: Biosimilars
JUNE 27, 2023
Amongst his accomplishments, Law360 considered the role James has played in leveraging little-used pathways to FDA approval for often first-ever drugs to treat rare diseases (e.g., James was only one of five life sciences attorneys selected and the only food and drug lawyer to make the list. In addition to Mr.
The Pharma Data
DECEMBER 3, 2020
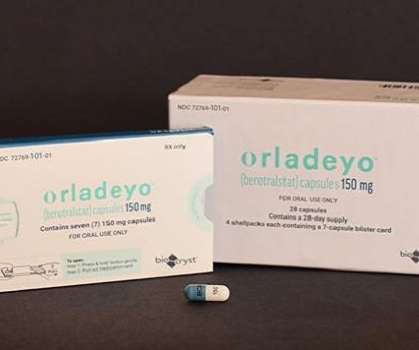
BioCryst Pharmaceuticals, Inc. Food and Drug Administration (FDA) has approved ORLADEYO (berotralstat) for prophylaxis to prevent attacks of hereditary angioedema (HAE) in adults and young patients 12 years and older. announced on Thursday that the U.S.

The Pharma Data
DECEMBER 16, 2020
–( BUSINESS WIRE )– ANI Pharmaceuticals, Inc. , (“ANI” or the “Company”) (Nasdaq: ANIP) today announced U.S. Food and Drug Administration (FDA) approval and the launch of Aminocaproic Acid Tablets USP, 500mg. market for this product is approximately $12.7 ANI Pharmaceuticals, Inc. BAUDETTE, Minn.–(

Policy Prescription
OCTOBER 3, 2023
Salant’s paper supports personal drug importation and legalizing the importation of prescription drugs for commercial use so that wholesale pharmacies, including companies like Amazon and Costco, could tap into the parallel importation markets of other countries. drug market is connected to foreign markets with lower drug prices.

The Pharma Data
JULY 8, 2021
is a leading global pharmaceutical company headquartered in Japan. Leveraging the experience gained from the development and marketing of a treatment for Alzheimer’s disease, Eisai aims to establish the “Eisai Dementia Platform.”

The Pharma Data
MAY 7, 2021
BioNTech is the Marketing Authorization Holder in the European Union, and the holder of emergency use authorizations or equivalent in the United States (together with Pfizer), United Kingdom, Canada and other countries in advance of a planned application for full marketing authorizations in these countries.

The Pharma Data
JANUARY 19, 2021
19, 2021 /PRNewswire/ — Regeneron Pharmaceuticals, Inc. financial markets open. SOURCE Regeneron Pharmaceuticals, Inc. TARRYTOWN, N.Y. , NASDAQ: REGN) today announced that it will report its fourth quarter and full year 2020 financial and operating results on Friday, February 5, 2021 , before the U.S. Source link.

The Pharma Data
JANUARY 13, 2021
“Receiving Fast Track designation is an important acknowledgment of the results of our COVID-19 laboratory research,” commented Leo Ehrlich, Chief Executive Officer at Innovation Pharmaceuticals. Alerts Sign-up for Innovation Pharmaceuticals email alerts is available at: [link]. Source link.

FDA Law Blog: Biosimilars
APRIL 21, 2024
Taiho Pharmaceutical Co., Usually at this point in a post we would identify the date of approval of the relevant NDA. But that’s the controversy here: Did FDA approve LYTGOBI NDA 214801 on September 30, 2022 when the Agency issued its initial approval letter , or on October 5, 2022 when FDA issued a corrected approval letter ?

The Pharma Data
APRIL 28, 2023
Teva and MedinCell Announce FDA Approval of UZEDY™ (risperidone) Extended-Release Injectable Suspension, a Long-Acting Subcutaneous Atypical Antipsychotic Injection, for the Treatment of Schizophrenia in Adults Teva Pharmaceuticals, a U.S. affiliate of Teva Pharmaceutical Industries Ltd.

The Pharma Data
DECEMBER 28, 2020
29, 2020 (GLOBE NEWSWIRE) — Amphastar Pharmaceuticals, Inc. , (NASDAQ: AMPH) announced that the U.S. Food and Drug Administration (“FDA”) has approved its Abbreviated New Drug Application (“ANDA”) for Glucagon for Injection Emergency Kit, 1 mg. RANCHO CUCAMONGA, Calif., ” According to IQVIA, the U.S.

The Pharma Data
JUNE 22, 2023
Paulsen, who joined Ferring’s Board of Directors 1 in July 2021, played a leading role in securing the US approval of Adstiladrin ® (nadofaragene firadnovec-vncg) a first-in-class gene therapy offering a new approach to treating a severe form of BCG-unresponsive non-muscle invasive bladder cancer. Ad hoc announcement pursuant to Art.

FDA Law Blog: Biosimilars
JANUARY 4, 2024
This unique forum, designed for in-house counsel and executives, as well as private practice attorneys working for the OTC drug industry will provide invaluable insights on FDA’s most recent directives and compliance standards governing OTC drug production, marketing and distribution.
The ChEMBL-og
FEBRUARY 22, 2021
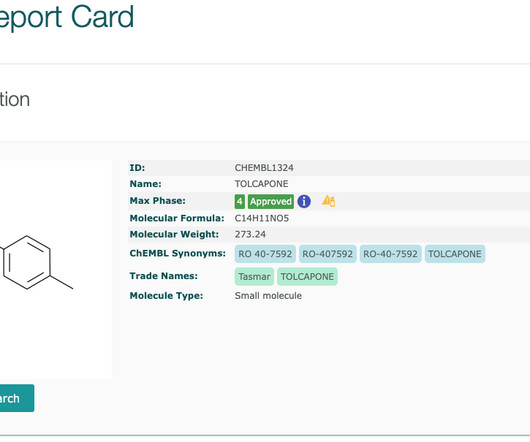
Boxed warnings (also know as black box warnings) are provided on medicinal product labels for FDA approved drugs if the medicinal product can cause severe or life-threatening side effects. For medicinal products that contain one active pharmaceutical ingredient, a boxed warning can be directly linked to a drug.

The Connected Lab
APRIL 8, 2020
The cost to develop a new prescription medicine that gains market approval has gone up 145% to $2.6 For patients suffering from an illness with no approved treatment, the wait can be unnerving. To this day, more than 400 million people suffer from rare diseases and 95% of rare diseases lack an FDA approved treatment 3.

The Pharma Data
JUNE 17, 2022
“By committing to bring biosimilar formulations such as Hyrimoz citrate-free HCF to patients, we are serving a critical need in expanding access to important medicines and fueling pharmaceutical innovation.”. The adalimumab reference medicine (Humira ®* ) was first approved with an adalimumab concentration of 50 mg/mL.

The Pharma Data
DECEMBER 4, 2021
–(BUSINESS WIRE)– Teva Pharmaceuticals, a U.S. affiliate of Teva Pharmaceutical Industries Ltd. The launch of our first-to-market authorized generic version of Epiduo® Forte Gel in the U.S. You are encouraged to report side effects of prescription drugs to the FDA. Teva Pharmaceutical Industries Ltd.

Advarra
AUGUST 16, 2022
Food and Drug Administration (FDA) approval. Since then, the FDA has significantly changed its approach to rare and orphan diseases. The FDA Since 1983. The Orphan Drug Act of 1983 was instrumental in changing the number of orphan drugs approved in the U.S.

PerkinElmer
JANUARY 7, 2022
The pharmaceutical industry is under huge pressure to address the high attrition rates in drug development. Furthermore, nearly one third of drugs get withdrawn from the market post approval due to safety concerns. link] New safety concerns identified for 1 in 3 FDA-approved drugs [Internet].
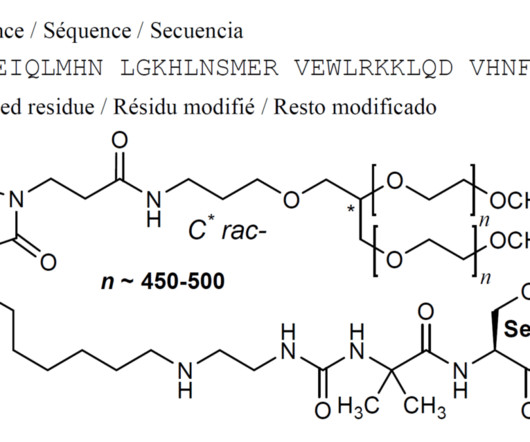
New Drug Approvals
SEPTEMBER 8, 2024
1] Palopegteriparatide was approved for medical use in the European Union in November 2023, [2] and in the United States in August 2024. [1] 5] The FDA granted the application for palopegteriparatide orphan drug and priority review designations. [5] Food and Drug Administration (FDA) (Press release). 14 August 2024.

Alta Sciences
FEBRUARY 8, 2024
Webinar: Assessing Cognition Impairment Using Driving Simulators in Clinical Trials pmjackson Thu, 02/08/2024 - 21:18 Over 20,000 FDA-approved drugs are currently on the market, and many contain psychoactive or sedative pharmaceutical ingredients that can alter the ability to operate a motor vehicle, making reducing the incidence of motor vehicle accidents (..)

The Pharma Data
NOVEMBER 29, 2020
Vanda Pharmaceuticals’ Hetlioz for Smith-Magenis Syndrome. Vanda Pharmaceuticals has a target action date of December 1 for the capsules and liquid formulation of Hetlioz for adults and children—the capsule formulation is a supplemental NDA (sNDA) and the liquid formulation is an NDA. It is approved in the U.S.
The Pharma Data
MARCH 26, 2021
If approved by the European Commission, ponesimod has the potential to help more people living with relapsing forms of MS.”. Despite continuous innovations in the treatment landscape, unmet needs remain. Damage to myelin slows or halts nerve conduction, producing the neurologic signs and symptoms of MS. [8].

The Pharma Data
JUNE 28, 2021
There are currently no FDA-approved anticoagulation therapies for pediatric patients with congenital heart disease who have undergone the Fontan procedure. About Rivaroxaban (Xarelto ) Rivaroxaban is the most broadly indicated non-vitamin K antagonist oral anticoagulant (NOAC) worldwide and is marketed under the brand name Xarelto.

The Pharma Data
NOVEMBER 5, 2020
05, 2020 (GLOBE NEWSWIRE) — Collegium Pharmaceutical, Inc. Nasdaq: COLL), a specialty pharmaceutical company committed to being the leader in responsible pain management, today reported its financial results for the quarter ended September 30, 2020 and provided a corporate update. FDA approval, and customary exceptions).

The Pharma Data
NOVEMBER 3, 2020
DEXTENZA is FDA approved for the treatment of ocular inflammation and pain following ophthalmic surgery. Ocular Therapeutix’s first commercial drug product, DEXTENZA, is FDA-approved for the treatment of ocular inflammation and pain following ophthalmic surgery. About DEXTENZA. Ocular Therapeutix, Inc.

The Pharma Data
DECEMBER 8, 2020
The fully integrated pharmaceutical company creates value through China’s specialty pharmaceutical markets with focus on iron deficiency, pain management and respiratory. SciNeuro Pharmaceuticals . Cornering the untapped Chinese CNS market, SciNeuro launched with $100 million in their pocket. Nuance Pharma .

The Pharma Data
APRIL 4, 2023
The approval includes all indications covered by the reference medicine*: rheumatic diseases, Crohn’s disease, ulcerative colitis, plaque psoriasis, uveitis and hidradenitis suppurativa. It has a leading global portfolio with eight marketed biosimilars and a further 15+ in various stages of development.

The Pharma Data
JANUARY 5, 2021
based contract manufacturing business, Benuvia Manufacturing, which has significant chemistry and formulation capabilities, including manufacturing our FDA-approved cannabinoid drug, SYNDROS ® ,” said Todd C. We look forward to supporting Radius through our U.S. Davis, executive chairman of Benuvia. Disease Highlights.

The Pharma Data
APRIL 11, 2023
affiliate of Teva Pharmaceutical Industries Ltd. affiliate of Teva Pharmaceutical Industries Ltd. About Teva Teva Pharmaceutical Industries Ltd. Around 200 million people around the world take a Teva medicine every day, and are served by one of the largest and most complex supply chains in the pharmaceutical industry.

Pharmaceutical Development Group
OCTOBER 8, 2022
Post EUA documents: EUA issuance does not imply approval or approval from the FDA. You will need the appropriate FDA approval or approval to continue commercializing your product. Getting GMP certification is beneficial to your company’s marketing and reputation.

The Pharma Data
DECEMBER 28, 2020
Nasdaq: MBRX) (Moleculin or the Company), a clinical stage pharmaceutical company with a broad portfolio of drug candidates targeting highly resistant tumors and viruses, today announced that the US Food and Drug Administration (FDA) has granted Orphan Drug Designation (ODD) to Annamycin for treatment of soft tissue sarcomas. .
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content