Topical Gene Therapy FDA-Approved for Severe Skin Disease, Dystrophic Epidermolysis Bullosa
PLOS: DNA Science
JUNE 1, 2023
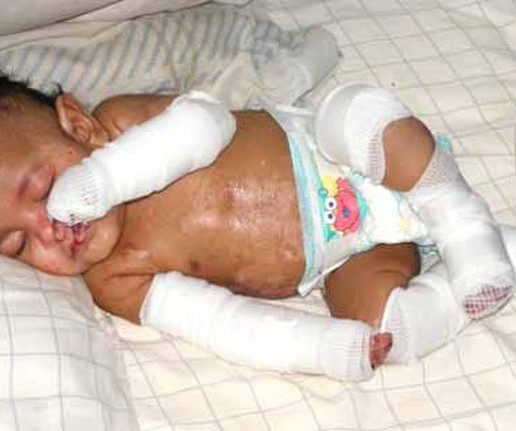
The newest FDA-approved gene therapy treats the severe, skin-peeling condition dystrophic epidermolysis bullosa (DEB). The gene treatment has been a long time coming, but it differs from the handful of other approved gene therapies: it isn’t a one-and-done. Primary investigator M.






























Let's personalize your content