Pfizer wins FDA approval for its $7B colitis drug
BioPharma Drive: Drug Pricing
OCTOBER 13, 2023
Acquired with the buyout of Arena Pharmaceuticals, Velsipity enters a competitive market for ulcerative colitis pills.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

BioPharma Drive: Drug Pricing
OCTOBER 13, 2023
Acquired with the buyout of Arena Pharmaceuticals, Velsipity enters a competitive market for ulcerative colitis pills.

Drugs.com
JANUARY 31, 2025
Food and Drug Administration (FDA) has approved Journavx, a new pain reliever without the risks of addiction or overdose linked to drugs like Vicodin and OxyContin.The new pill, developed by Vertex Pharmaceuticals.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Drug Patent Watch
JULY 24, 2024
In the high-stakes world of pharmaceuticals, generic drugs have become the unsung heroes of healthcare accessibility. These cost-effective alternatives to […] Source

SCIENMAG: Medicine & Health
OCTOBER 31, 2023
— A new drug developed by professors from the School of Pharmacy and Pharmaceutical Sciences at Binghamton University has received Food and Drug Administration (FDA) approval for the treatment of patients with Duchenne muscular dystrophy (DMD), a common genetic disease that mostly affects young boys. BINGHAMTON, N.Y.
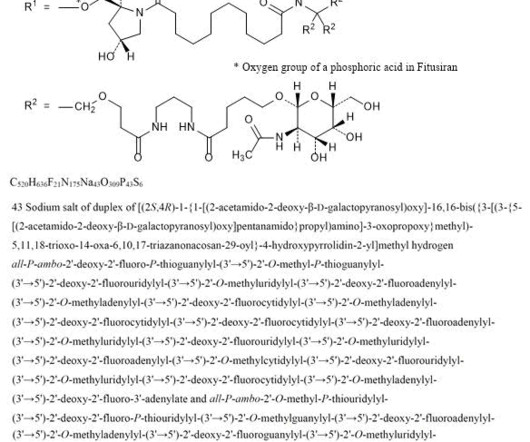
New Drug Approvals
APRIL 11, 2025
2] Fitusiran was approved for medical use in the United States in March 2025. [2] 2] The fixed dose of fitusiran is not approved because it led to excessive clotting in some participants. [2] 2] The US Food and Drug Administration (FDA) granted the application for fitusiran orphan drug and fast track designations. 26 March 2025.

The Pharma Data
AUGUST 27, 2020
The test is FDA-approved to report short variants in 311 genes including rearrangements and copy number losses in BRCA1 and BRCA2 genes. The results are delivered in an integrated report that identifies alterations matched to FDA-approved therapies.
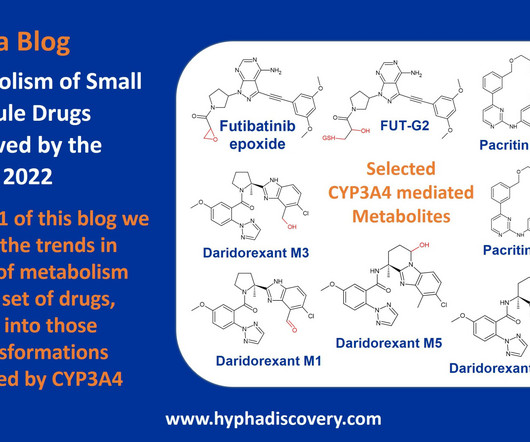
Metabolite Tales Blog
JANUARY 26, 2023
Metabolism of 2022 FDA approved small molecule drugs – Part 1 Does CYP3A4 still rule? By Julia Shanu-Wilson It won’t come as much surprise to learn that of the 17 small molecules* approved by the FDA in 2022, CYP3A4 was the major player in drug metabolism. References Iversen et al., Front Pharmacol.,

The Pharma Data
NOVEMBER 2, 2020
FDA Approves Bronchitol (mannitol) Inhalation Powder to Improve Pulmonary Function in Adult Patients with Cystic Fibrosis. Food and Drug Administration (FDA) approval of Bronchitol (mannitol) inhalation powder. Food and Drug Administration (FDA) approval of Bronchitol (mannitol) inhalation powder.

Agency IQ
JANUARY 12, 2024
After a years-long review process, the FDA today announced its approval of Florida’s proposal to import certain prescription drug products from Canada. Fill out the form to read the full article.

The Pharma Data
NOVEMBER 27, 2020
FDA Approves Danyelza (naxitamab-gqgk) for the Treatment of Neuroblastoma. It’s very exciting to see this treatment go from being an experimental therapy used at my daughter’s bedside to now being FDA approved. Related Articles: Danyelza (naxitamab-gqgk) FDA Approval History. NEW YORK, Nov. Source link.

The Pharma Data
DECEMBER 16, 2020
17, 2020 /PRNewswire/ — CNS Pharmaceuticals, Inc. We will now rapidly move to initiate our Phase 2 trial of Berubicin for adults with GBM and expect to begin enrolling patients in the first quarter of next year,” commented John Climaco , CEO of CNS Pharmaceuticals. About CNS Pharmaceuticals, Inc.

The Pharma Data
NOVEMBER 27, 2020
FDA Approves Imcivree (setmelanotide) for Chronic Weight Management in Patients with Obesity Due to POMC, PCSK1 or LEPR Deficiency. 27, 2020 (GLOBE NEWSWIRE) — Rhythm Pharmaceuticals, Inc. With this approval, Imcivree becomes the first-ever FDA approved therapy for these rare genetic diseases of obesity.

Pharmaceutical Development Group
AUGUST 9, 2021
Formulation chemistry is the systematic and step-by-step approach to pharmaceutical development. Nowadays, as the usage of medicines is increasing, pharmaceutical companies are more eager to bring manufacturing a new look in quality and performance. There are different tests to assess the quality of a pharmaceutical product.

The Pharma Data
DECEMBER 15, 2020
FDA Approves Klisyri (tirbanibulin) for the Treatment of Actinic Keratosis on the Face or Scalp. Food and Drug Administration (FDA) has approved Klisyri (tirbanibulin) for the topical treatment of actinic keratosis (AK) on the face or scalp. The FDA approval of Klisyri is a significant milestone for Athenex.

FDA Law Blog: Biosimilars
OCTOBER 1, 2024
Karst — If you monitor Regulations.gov dockets and litigation dockets on PACER like we do, then you know that one company name—more than any other over the past several years—pops up: Vanda Pharmaceuticals, Inc. VANDA PHARMACEUTICALS, INC. VANDA PHARMACEUTICALS, INC. 24-270 VANDA PHARMACEUTICALS, INC. 23-5200 (D.C.

The Pharma Data
MAY 27, 2022
Novartis today announced the US Food and Drug Administration (FDA) has granted accelerated approval for Kymriah ® (tisagenlecleucel) for the treatment of adult patients with relapsed or refractory (r/r) follicular lymphoma (FL) after two or more lines of systemic therapy.

Advarra
JULY 27, 2022
As a pharmaceutical product makes its way through the lifecycle, there are often Food and Drug Administration (FDA) guidelines organizations should pay particular attention to. If your go-to-market strategy requires FDA approval, it may also require a prior approval supplement. Go-to-market Strategy.

Drug Target Review
JANUARY 8, 2024
Jerry has over 30 years of experience in the biopharmaceutical industry and has been involved in the discovery, clinical development, and global commercialisation of more than a dozen FDA-approved drugs with multiple successful exits. a commercial stage pharmaceutical company. acquired by Teva Pharmaceuticals in 2014.

The Pharma Data
OCTOBER 21, 2020
Food and Drug Administration (FDA) to market Ephedrine Sulfate Injection in a ready-to-use 50mg/10 ml single use vial presentation. Under an exclusive licensing agreement with Endo International’s (NASDAQ: ENDP) subsidiary, Endo Ventures Limited, Par Pharmaceuticals’ Sterile Products division will launch and distribute the product.

The Pharma Data
DECEMBER 1, 2020
VICTORIA, British Columbia–( BUSINESS WIRE )– Aurinia Pharmaceuticals Inc. Aurinia Pharmaceuticals is a late-stage clinical biopharmaceutical company focused on developing and commercializing therapies to treat targeted patient populations that are impacted by serious diseases with a high unmet medical need. ABOUT AURINIA.

The Pharma Data
APRIL 19, 2022
Food and Drug Administration (FDA) has approved commercial production at the company’s new CAR T-cell therapy manufacturing facility in Frederick, Maryland. The site will produce Kite’s FDA approved CAR T-cell therapy used to treat blood cancer.

The Pharma Data
JANUARY 7, 2021
VICTORIA, British Columbia–( BUSINESS WIRE )– Aurinia Pharmaceuticals Inc. Aurinia Pharmaceuticals is a late-stage clinical biopharmaceutical company focused on developing and commercializing therapies to treat targeted patient populations that are impacted by serious diseases with a high unmet medical need. ABOUT AURINIA.
New Drug Approvals
APRIL 2, 2025
1] [2] It was developed by Vertex Pharmaceuticals , [5] and was approved for medical use in the United States in January 2025. [2] 2] [6] Suzetrigine is the first medication to be approved by the US Food and Drug Administration (FDA) in this new class of pain management medicines. [2] Food and Drug Administration (FDA).

The Pharma Data
AUGUST 31, 2020
Food and Drug Administration (FDA) approval for the cobas® HIV-1/HIV-2 Qualitative Test for use on the fully automated cobas® 6800/8800 Systems in the U.S. About Roche Roche is a global pioneer in pharmaceuticals and diagnostics focused on advancing science to improve people’s lives. Learn more now: www.cobas68008800.com.
The Pharma Data
JULY 29, 2021
The FDA released new materials for health care providers to enhance understanding about biosimilar and interchangeable biosimilar products, including a fact sheet about interchangeable biosimilar products. The FDA granted approval of Semglee (insulin glargine-yfgn) to Mylan Pharmaceuticals Inc. Source link: [link].

DrugBank
DECEMBER 18, 2024
A new shift is occurring in the pharmaceutical industry, leading to a rapidly expanding field known as digital therapeutics (DTx). DTx interventions include sensor-equipped wearable devices, remote patient monitoring tools, and virtual reality platforms integrated with conventional pharmaceutical treatments.
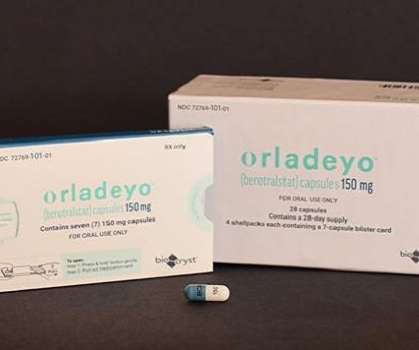
The Pharma Data
DECEMBER 3, 2020
BioCryst Pharmaceuticals, Inc. Food and Drug Administration (FDA) has approved ORLADEYO (berotralstat) for prophylaxis to prevent attacks of hereditary angioedema (HAE) in adults and young patients 12 years and older. announced on Thursday that the U.S.
Drug Target Review
MARCH 12, 2024
Scientists from the Skaggs School of Pharmacy and Pharmaceutical Sciences at the University of California San Diego have discovered thousands of bile acids. The study’s findings offer new insights into the biochemical language microbes use to influence distant organ systems, which could revolutionise the way researchers approach disease.

Pharmaceutical Development Group
DECEMBER 19, 2021
Start Up and Generic Pharmaceutical Drug and Biologic Companies have high quality, affordable products that improve the quality of life for their patients. In a FDA Pre-Submission that leads to FDA Approval, more does not equal better and more does not equal relevance to a specific Pharmaceutical Drug or Biologic Product.

Pharmaceutical Development Group
JANUARY 7, 2022
Start Up and Generic Pharmaceutical Drug and Biologic Companies have high quality, affordable products and biosimilars that improve the quality of life for their patients. Dr. Spanogle has taken scores of Regulated Life Science Products from discovery to approval and launch; and audited, identified, and remediated QMS deficiencies.

FDA Law Blog: Biosimilars
JUNE 27, 2023
Amongst his accomplishments, Law360 considered the role James has played in leveraging little-used pathways to FDA approval for often first-ever drugs to treat rare diseases (e.g., James was only one of five life sciences attorneys selected and the only food and drug lawyer to make the list.
The Pharma Data
NOVEMBER 2, 2020
GW Pharmaceuticals hopes to bring its cannabis-based treatment for multiple sclerosis spasticity to the United States. Sativex is approved for use in parts of Europe for this indication. Justin Gover, chief executive officer of GW Pharmaceuticals, proclaimed his excitement about launching the Phase III study in the United States.

The Pharma Data
JULY 8, 2021
is a leading global pharmaceutical company headquartered in Japan. We routinely post information that may be important to investors on our website at www.biogen.com. Follow us on social media – Twitter , LinkedIn , Facebook , YouTube. About Eisai Co.,

The Pharma Data
JANUARY 6, 2021
SK Chemicals’ life sciences business is dominated by pharmaceuticals and biologics and is aimed at providing comprehensive healthcare solutions that cover patient care from diagnosis to treatment and prevention.

The Pharma Data
MAY 31, 2022
In recognizing our endeavor to pursue a long-term perspective in all we do, Roche has been named one of the most sustainable companies in the pharmaceuticals industry by the Dow Jones Sustainability Indices for the thirteenth consecutive year.

The Pharma Data
DECEMBER 16, 2020
–( BUSINESS WIRE )– ANI Pharmaceuticals, Inc. , (“ANI” or the “Company”) (Nasdaq: ANIP) today announced U.S. Food and Drug Administration (FDA) approval and the launch of Aminocaproic Acid Tablets USP, 500mg. ANI Pharmaceuticals, Inc. BAUDETTE, Minn.–( The current annual U.S. earlier this year.

New Drug Approvals
DECEMBER 19, 2022
FDA 12/1/2022, To treat adults with relapsed or refractory acute myeloid leukemia with a susceptible isocitrate dehydrogenase-1 (IDH1) mutation, Rezlidhia Olutasidenib , sold under the brand name Rezlidhia , is an anticancer medication used to treat relapsed or refractory acute myeloid leukemia. [1] Rigel Pharmaceuticals. Hz, 1 H), 4.62−4.75

The Pharma Data
JANUARY 6, 2021
UNION therapeutics A/S is a privately held, clinical stage, pharmaceutical company dedicated to the development of novel treatments for inflammatory and infectious diseases. About UNION therapeutics A/S.

The Pharma Data
DECEMBER 16, 2020
16, 2020 /PRNewswire/ — Regeneron Pharmaceuticals, Inc. Founded and led for over 30 years by physician-scientists, our unique ability to repeatedly and consistently translate science into medicine has led to eight FDA-approved treatments and numerous product candidates in development, all of which were homegrown in our laboratories.
KIF1A
JULY 1, 2023
Taking a drug that shelved during development or didn’t receive FDA approval, and assessing its application for another disease, is called drug repositioning. It requires a deep look at former products that ended at various stages of research and development.

The Pharma Data
APRIL 28, 2023
Teva and MedinCell Announce FDA Approval of UZEDY™ (risperidone) Extended-Release Injectable Suspension, a Long-Acting Subcutaneous Atypical Antipsychotic Injection, for the Treatment of Schizophrenia in Adults Teva Pharmaceuticals, a U.S. affiliate of Teva Pharmaceutical Industries Ltd.
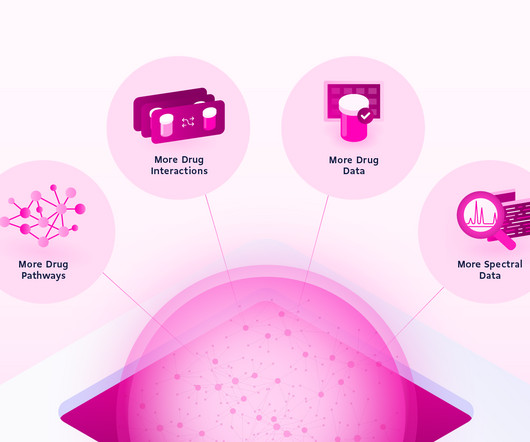
DrugBank
FEBRUARY 1, 2024
There’s a lot to dig into so we’ve highlighted a few improvements we think are really going to change the game: 300% Increase in Drug-Drug Interaction Data Navigating the complex world of pharmaceuticals can be daunting. DrugBank 6.0 simplifies this with a significant expansion in our drug-drug interaction data.

The Pharma Data
MAY 7, 2021
BioNTech has established a broad set of relationships with multiple global pharmaceutical collaborators, including Genmab, Sanofi, Bayer Animal Health, Genentech, a member of the Roche Group, Regeneron, Genevant, Fosun Pharma, and Pfizer. For more information, please visit www.BioNTech.de.

The Pharma Data
JANUARY 19, 2021
19, 2021 /PRNewswire/ — Regeneron Pharmaceuticals, Inc. Founded and led for over 30 years by physician-scientists, our unique ability to repeatedly and consistently translate science into medicine has led to eight FDA-approved treatments and numerous product candidates in development, all of which were homegrown in our laboratories.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content