Binghamton researchers get FDA approval for drug to treat world’s most common genetic disease
SCIENMAG: Medicine & Health
OCTOBER 31, 2023
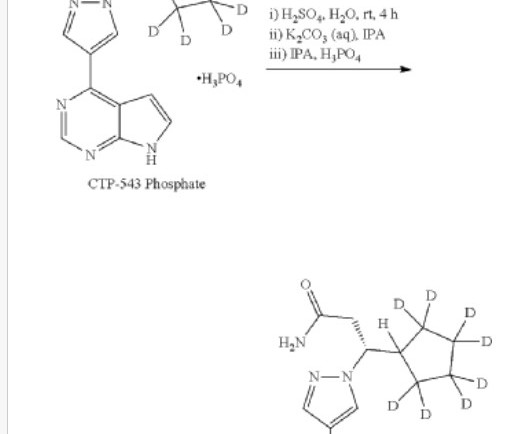
— A new drug developed by professors from the School of Pharmacy and Pharmaceutical Sciences at Binghamton University has received Food and Drug Administration (FDA) approval for the treatment of patients with Duchenne muscular dystrophy (DMD), a common genetic disease that mostly affects young boys. BINGHAMTON, N.Y.











































Let's personalize your content