FDA approves Novartis Kymriah® CAR-T cell therapy for adult patients with relapsed or refractory follicular lymphoma
The Pharma Data
MAY 27, 2022
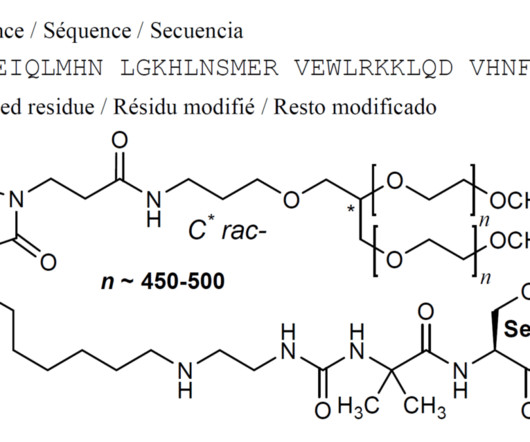
Novartis today announced the US Food and Drug Administration (FDA) has granted accelerated approval for Kymriah ® (tisagenlecleucel) for the treatment of adult patients with relapsed or refractory (r/r) follicular lymphoma (FL) after two or more lines of systemic therapy.











































Let's personalize your content