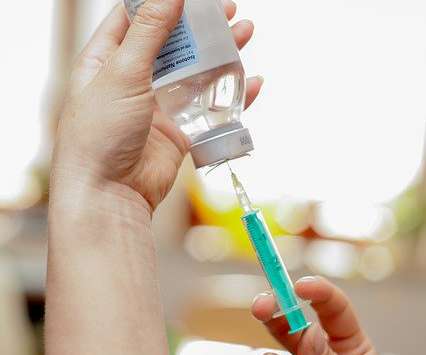
First-in-human clinical trial for a vaccine to treat opioid use.
The Pharma Data
SEPTEMBER 7, 2021
The first patients have been enrolled in a phase 1 randomized placebo-controlled clinical trial to study a therapeutic vaccine for opioid use disorder developed by researchers at the University of Minnesota Medical School. The vaccine currently being tested stimulates the body’s immune system to produce antibodies to oxycodone.



















Let's personalize your content