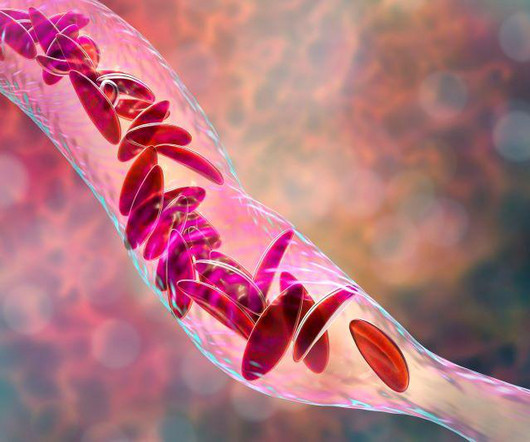
FDA Approves Landmark Sickle Cell Gene Therapies, Casgevy and Lyfgenia
Drugs.com
DECEMBER 8, 2023
Food and Drug Administration on Friday approved two milestone gene therapies for sickle cell disease, including the first treatment ever approved that uses gene-editing technology. FRIDAY, Dec. 8, 2023 -- The U.S. Casgevy, developed by Vertex.








































Let's personalize your content