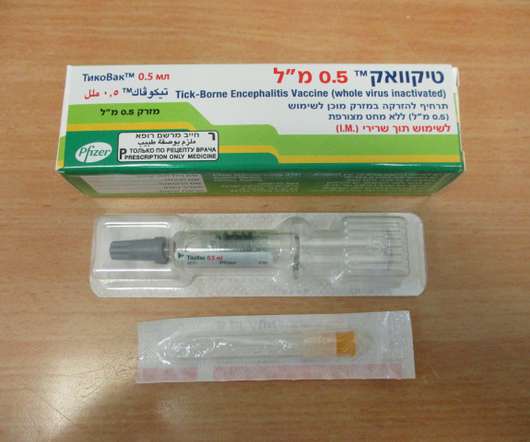
U.S. FDA APPROVES TICOVAC™, PFIZER’S TICK-BORNE ENCEPHALITIS (TBE) VACCINE
The Pharma Data
AUGUST 15, 2021
Food and Drug Administration (FDA) has approved TICOVAC (tick-borne encephalitis (TBE) vaccine) for active immunization to prevent TBE in individuals 1 year of age and older. 1 TICOVAC is the only FDA-approved vaccine to help protect U.S. Following today’s FDA approval, the U.S. 45 years ago.












































Let's personalize your content