Pfizer wins FDA approval of new meningococcal vaccine
BioPharma Drive: Drug Pricing
OCTOBER 23, 2023
The clearance of the pentavalent shot Penbraya adds to Pfizer’s infectious disease portfolio as it adjusts to slumping COVID-19 vaccine sales.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

BioPharma Drive: Drug Pricing
OCTOBER 23, 2023
The clearance of the pentavalent shot Penbraya adds to Pfizer’s infectious disease portfolio as it adjusts to slumping COVID-19 vaccine sales.

Drugs.com
FEBRUARY 18, 2025
Food and Drug Administration has approved Penmenvy (Meningococcal Groups A, B, C, W, and Y Vaccine) for active immunization against invasive meningococcal disease (IMD), according to a press release from. TUESDAY, Feb. 18, 2025 -- The U.S.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drugs.com
SEPTEMBER 20, 2024
Food and Drug Administration on Friday approved FluMist nasal spray as the first influenza vaccine that can be. FRIDAY, Sept. 20, 2024 -- The days of waiting for a flu shot at your doctor's office or local pharmacy may be over: The U.S.

Drugs.com
SEPTEMBER 20, 2024
Food and Drug Administration on Friday approved FluMist nasal spray as the first influenza vaccine that can be. FRIDAY, Sept. 20, 2024 -- The days of waiting for a flu shot at your doctor's office or local pharmacy may be over: The U.S.

Drugs.com
NOVEMBER 10, 2023
10, 2023 (Healthday News) -- The first vaccine to prevent infection with the chikungunya virus was approved by the U.S. The single-dose shot, known as Ixchiq, is approved for adults who are at. FRIDAY, Nov. Food and Drug Administration on Thursday.

Drugs.com
AUGUST 22, 2023
22, 2023 -- Women may soon have a vaccine they can take during a pregnancy to help protect their newborn from respiratory syncytial virus (RSV), following U.S. Food and Drug Administration approval of the shot, called Abrysvo, on. TUESDAY, Aug.

Drugs.com
FEBRUARY 18, 2025
Food and Drug Administration has approved Penmenvy (Meningococcal Groups A, B, C, W, and Y Vaccine) for active immunization against invasive meningococcal disease (IMD), according to a press release from. TUESDAY, Feb. 18, 2025 -- The U.S.

Drugs.com
AUGUST 22, 2023
22, 2023 -- Women may soon have a vaccine they can take during a pregnancy to help protect their newborn from respiratory syncytial virus (RSV), following U.S. Food and Drug Administration approval of the shot, called Abrysvo, on. TUESDAY, Aug.

The Pharma Data
OCTOBER 30, 2020
Dive into this week’s update for more details on the actions taken by the FDA in the ongoing response to the Covid-19 pandemic. FDA approves first treatment for Covid-19. On October 22, the FDA approved the antiviral drug Veklury for use in adult and pediatric patients for the treatment of Covid-19 requiring hospitalization.

The Pharma Data
MAY 7, 2021
The Pfizer-BioNTech COVID-19 Vaccine is currently available in the U.S. under an Emergency Use Authorization (EUA) granted by the FDA on December 11, 2020. Since then, the companies have delivered more than 170 million doses of the vaccine across the U.S. We are pleased to work with U.S. AUTHORIZED USE IN THE U.S.:

The Pharma Data
SEPTEMBER 23, 2020
The FDA is set to release new guidelines that would raise safety and efficacy requirements required for a COVID-19 vaccine to be granted Emergency Use Authorization. . The FDA will also assess at least five severe coronavirus patients in the placebo group of each trial. This has been revealed by The Washington Post.

The Pharma Data
NOVEMBER 16, 2020
November 16, 2020 — An independent data and safety monitoring board (DSMB) overseeing the Phase 3 trial of the investigational COVID-19 vaccine known as mRNA-1273 reviewed trial data and shared its interim analysis with the trial oversight group on Nov. The interim analysis comprised 95 cases of symptomatic COVID-19 among volunteers.

The Pharma Data
MAY 4, 2021
Pfizer plans to file for full FDA approval of Covid vaccine at the end of this month ( CNBC ). The FDA is set to authorize the Pfizer-BioNTech vaccine for those 12-15 years old by early next week. White House to shift COVID-19 vaccine to states with more need ( Reuters ).

The Pharma Data
JANUARY 29, 2021
29 January 2021 — AstraZeneca’s COVID-19 vaccine has been recommended for conditional marketing authorisation (CMA) in the European Union (EU) for active immunisation to prevent COVID-19 caused by SARS-CoV-2, in individuals 18 years of age and older.

The Pharma Data
JANUARY 29, 2021
29 January 2021 — AstraZeneca’s COVID-19 vaccine has been granted a conditional marketing authorisation (CMA) in the European Union (EU) for active immunisation to prevent COVID-19 caused by SARS-CoV-2, in individuals 18 years of age and older.

The Pharma Data
NOVEMBER 20, 2020
Food and Drug Administration has scheduled a meeting of its Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Dec. 10 to discuss the request for emergency use authorization (EUA) of a COVID-19 vaccine from Pfizer, Inc. Source: FDA. BNT162b2 (SARS-CoV-2 vaccine) FDA Approval History.
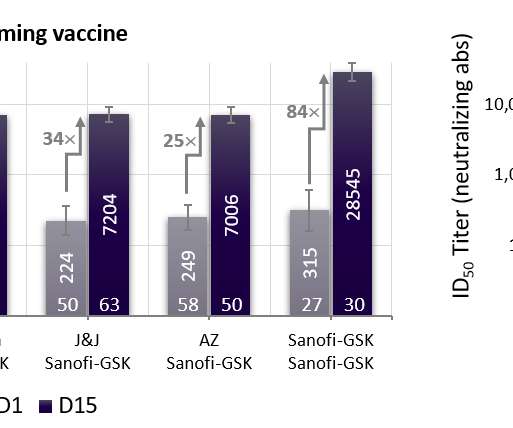
The Pharma Data
FEBRUARY 23, 2022
* Final analysis of the global VAT02 booster trial confirms universal ability to boost neutralizing antibodies 18- to 30-fold across vaccine platforms (mRNA, adenovirus). * efficacy against any symptomatic COVID-19 disease, in line with expected vaccine effectiveness in today’s environment dominated by variants of concern.

The Pharma Data
NOVEMBER 23, 2020
. Positive high-level results from an interim analysis of clinical trials of AZD1222 in the UK and Brazil showed the vaccine was highly effective in preventing COVID-19, the primary endpoint, and no hospitalisations or severe cases of the disease were reported in participants receiving the vaccine.
The Pharma Data
SEPTEMBER 7, 2021
The first patients have been enrolled in a phase 1 randomized placebo-controlled clinical trial to study a therapeutic vaccine for opioid use disorder developed by researchers at the University of Minnesota Medical School. The vaccine currently being tested stimulates the body’s immune system to produce antibodies to oxycodone.

The Pharma Data
NOVEMBER 30, 2020
Nasdaq: NVAX), a late-stage biotechnology company developing next-generation vaccines for serious infectious diseases, today provided an update on its COVID-19 vaccine program. Vaccines Taskforce and National Institute for Health Research played pivotal roles in the rapid recruitment and enrollment of volunteers. and Australia.

The Pharma Data
JANUARY 6, 2021
(Nasdaq: MRNA), a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines, today announced that the European Commission has granted a conditional marketing authorization (CMA) for COVID-19 Vaccine Moderna, allowing vaccination programs using the Moderna vaccine to be rolled out across the European Union.

The Pharma Data
DECEMBER 30, 2020
30 December 2020 — AstraZeneca’s COVID-19 vaccine has been approved for emergency supply in the UK, with the first doses being released today so that vaccinations may begin early in the New Year. This is the first authorisation for this vaccine.

The Pharma Data
NOVEMBER 9, 2020
Nasdaq: NVAX), a late-stage biotechnology company developing next-generation vaccines for serious infectious diseases, today announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track Designation for NVX-CoV2373, the Company’s COVID-19 vaccine candidate. GAITHERSBURG, Md., Glenn, M.D., and globally.”.

The Pharma Data
JANUARY 12, 2021
Nasdaq: MRNA), a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines, today announced that Swissmedic, the Swiss Agency for Therapeutic Products, has authorized the COVID-19 Moderna Vaccine in Switzerland. million doses of the COVID-19 Vaccine Moderna. About the COVID-19 Vaccine Moderna.

The Pharma Data
NOVEMBER 12, 2020
The World Health Organization (WHO)’s ACT-Accelerator program is focusing on increasing global access to dexamethasone and monoclonal antibodies and has built up significant vaccine manufacturing capabilities, but it has zero plans for facilitating access to the only FDA-approved COVID-19 treatment, remdesivir. Source link.
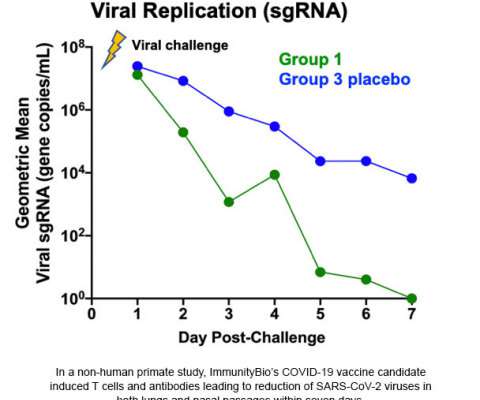
The Pharma Data
DECEMBER 10, 2020
–( BUSINESS WIRE )– ImmunityBio , a privately-held immunotherapy company, today announced its COVID-19 vaccine candidate protected nasal and lung airways of non-human primates against coronavirus (SARS-CoV-2) in a challenge study. 11, 2020 02:48 UTC. CULVER CITY, Calif.–(
The Pharma Data
AUGUST 16, 2021
Food and Drug Administration (FDA) to support the evaluation of a third, or booster, dose of the companies’ COVID-19 vaccine (BNT162b2) for future licensure. The data we’ve seen to date suggest a third dose of our vaccine elicits antibody levels that significantly exceed those seen after the two-dose primary schedule.

The Pharma Data
DECEMBER 3, 2020
The Phase 1 trial was a randomized, observer-blind, placebo-controlled study to assess the safety, reactogenicity and immunogenicity of the adjuvanted COVID-19 S-Trimer vaccine candidates formulated with different antigen levels. No serious adverse events related to the vaccine candidates studied were reported.

The Pharma Data
NOVEMBER 16, 2020
We believe that our investments in mRNA delivery technology and manufacturing process development will allow us to store and ship our COVID-19 vaccine candidate at temperatures commonly found in readily available pharmaceutical freezers and refrigerators,” said Juan Andres, Chief Technical Operations and Quality Officer at Moderna. “We

The Pharma Data
JANUARY 31, 2021
Adjuvanted S-Trimer COVID-19 vaccine candidates demonstrated favorable safety and tolerability profiles and strong neutralizing immune responses in a phase 1 trial. Clover plans to initiate a global phase 2/3 trial in the first half of 2021 with an interim analysis for vaccine efficacy potentially in the middle of 2021.

The Pharma Data
FEBRUARY 24, 2022
If the EC grants the variation, the decision will be immediately applicable to all 27 EU member states, making booster vaccines available to everyone 12 years and older. . The COVID-19 vaccine booster for individuals 12 through 15 years of age was already granted Emergency Use Authorization by the U.S. and Europe.

The Pharma Data
DECEMBER 11, 2020
FDA authorizes COVID-19 mRNA vaccine for emergency use; companies are prepared to deliver first doses in the U.S. In collaboration with Operation Warp Speed, Pfizer and BioNTech, as well as other vaccine companies are expected to deliver hundreds of millions of vaccine doses to Americans by the end of 2021.

The Pharma Data
NOVEMBER 20, 2020
billion doses by the end of 2021; the companies will be ready to distribute the vaccine within hours after authorization. BNT162b2 demonstrated a vaccine efficacy rate of 95%, with no serious safety concerns observed to date. by the middle to end of December 2020. participants are 56-85 years of age.

The Pharma Data
NOVEMBER 16, 2020
The primary endpoint of the Phase 3 COVE study is based on the analysis of COVID-19 cases confirmed and adjudicated starting two weeks following the second dose of vaccine. All 11 cases occurred in the placebo group and none in the mRNA-1273 vaccinated group. The majority of adverse events were mild or moderate in severity.

The Pharma Data
DECEMBER 8, 2020
Nasdaq: MRNA) a biotechnology company pioneering messenger RNA (mRNA) therapeutics and vaccines to create a new generation of transformative medicines for patients, today announced the Swiss Federal Government has increased its confirmed order commitment from 4.5 million doses of Moderna’s vaccine candidate against COVID-19 , mRNA-1273. “As

The Pharma Data
NOVEMBER 9, 2020
Vaccine candidate was found to be more than 90% effective in preventing COVID-19 in participants without evidence of prior SARS-CoV-2 infection in the first interim efficacy analysis. This means that protection is achieved 28 days after the initiation of the vaccination, which consists of a 2-dose schedule.

The Pharma Data
AUGUST 15, 2021
Food and Drug Administration (FDA) has approved TICOVAC (tick-borne encephalitis (TBE) vaccine) for active immunization to prevent TBE in individuals 1 year of age and older. 1 TICOVAC is the only FDA-approved vaccine to help protect U.S. Following today’s FDA approval, the U.S. 45 years ago.
The Pharma Data
DECEMBER 12, 2020
Food and Drug Administration approved Pfizer’s coronavirus vaccine for emergency use on Friday, clearing the way for the launch of a national campaign to inoculate enough Americans to stem the spread of COVID-19. ” Who is first in line to be vaccinated? SATURDAY, Dec. 12, 2020 – The U.S.

The Pharma Data
JUNE 18, 2021
First approval of a conjugate vaccine that helps protect against 20 serotypes responsible for the majority of invasive pneumococcal disease and pneumonia, 1,2,3,4,5,6,7 including seven responsible for 40% of pneumococcal disease cases and deaths in the U.S. Following today’s FDA approval, the U.S. Jansen, Ph.D.,

The Pharma Data
MARCH 29, 2022
Food and Drug Administration authorized a second booster dose of either the Pfizer-BioNTech or the Moderna COVID-19 vaccines for older people and certain immunocompromised individuals. The FDA previously authorized a single booster dose for certain immunocompromised individuals following completion of a three-dose primary vaccination series.

Drugs.com
AUGUST 22, 2024
22, 2024 -- Updated shots to shield against COVID-19 infection were approved by the U.S. Food and Drug Administration on Thursday.This year's approval for the updated mRNA vaccines comes much sooner than happened in 2023, when fall. THURSDAY, Aug.

The Pharma Data
JANUARY 21, 2021
We focus on areas of medicine where we can make the biggest difference: Cardiovascular & Metabolism, Immunology, Infectious Diseases & Vaccines, Neuroscience, Oncology, and Pulmonary Hypertension. Learn more at www.janssen.com and follow us at www.twitter.com/JanssenGlobal and www.twitter.com/JanssenUS.

Drugs.com
SEPTEMBER 11, 2023
Food and Drug Administration on Monday gave the green light to new COVID boosters for Americans, setting the stage for the updated vaccines to become available within days. MONDAY, Sept. 11, 2023 -- The U.S. The COVID-19 shots from Pfizer and.

The Pharma Data
DECEMBER 10, 2020
. Data from 43,448 participants, half of whom received BNT162b2 and half of whom received placebo, showed that the vaccine candidate was well tolerated and demonstrated 95% efficacy in preventing COVID-19 in those without prior infection 7 days or more after the second dose. Jansen, Ph.D.,
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content