FDA Warns of Toxic Lead in Cinnamon Products
Drugs.com
MARCH 6, 2024
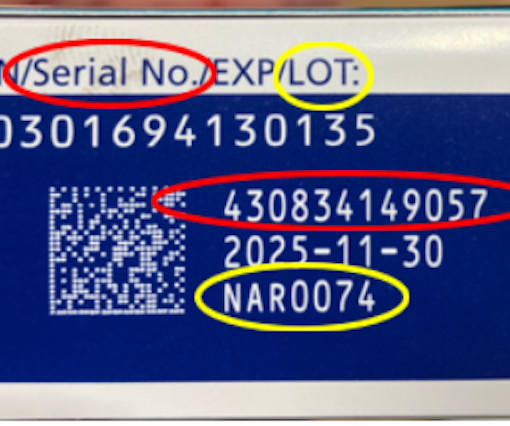
The FDA urged folks to throw away and not buy the following brands of. WEDNESDAY, March 6, 2024 -- The U.S. Food and Drug Administration issued a health advisory Wednesday warning consumers that six brands of ground cinnamon are tainted with lead.














































Let's personalize your content