FDA approves new CAR-T competitor to Gilead’s Tecartus
BioPharma Drive: Drug Pricing
NOVEMBER 11, 2024
Developer Autolus set a list price of $525,000 for its new cell therapy, which it will sell for a type of leukemia under the brand name Aucatzyl.
This site uses cookies to improve your experience. By viewing our content, you are accepting the use of cookies. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country we will assume you are from the United States. View our privacy policy and terms of use.

BioPharma Drive: Drug Pricing
NOVEMBER 11, 2024
Developer Autolus set a list price of $525,000 for its new cell therapy, which it will sell for a type of leukemia under the brand name Aucatzyl.
Drugs.com
DECEMBER 8, 2023
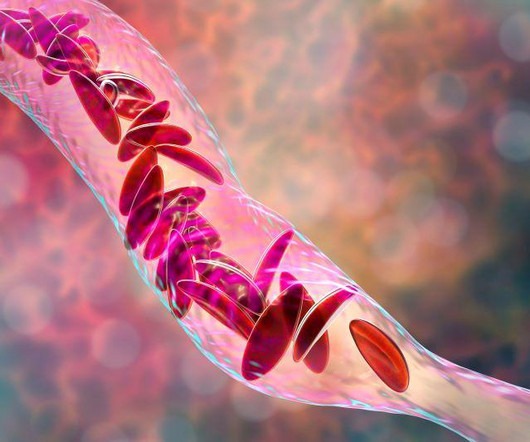
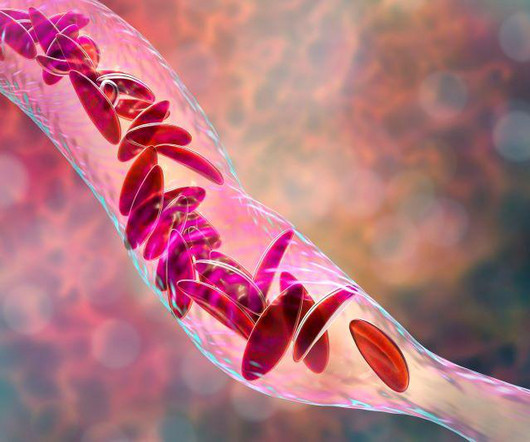
Food and Drug Administration on Friday approved two milestone gene therapies for sickle cell disease, including the first treatment ever approved that uses gene-editing technology.Casgevy, developed by Vertex. FRIDAY, Dec. 8, 2023 -- The U.S.
Drugs.com
DECEMBER 8, 2023
Food and Drug Administration on Friday approved two milestone gene therapies for sickle cell disease, including the first treatment ever approved that uses gene-editing technology. FRIDAY, Dec. 8, 2023 -- The U.S. Casgevy, developed by Vertex.

PLOS: DNA Science
MARCH 28, 2024
FDA classifies it as a “nonsteroidal treatment” – not a gene therapy, but it affects gene expression. Results from the study that led to the FDA approval appeared in The Lancet Neurology in April 2024 with commentary. Researchers have been working on developing gene therapy for DMD for decades.

FDA Law Blog: Drug Discovery
MARCH 26, 2024
Sasinowski — On March 21st, FDA announced the approval of the first nonsteroidal therapy for the treatment of Duchenne Muscular Dystrophy (DMD) (FDA press release available here ). All the data described above can be found in Sections 12 and 14 of the FDA-approved prescribing information (see here ).

BioPharma Drive: Drug Pricing
JUNE 4, 2024
A group of independent experts wasn't convinced by clinical trial data from company Lykos Therapeutics, which is seeking FDA approval of MDMA-assisted treatment for post-traumatic stress disorder.

BioPharma Drive: Drug Pricing
FEBRUARY 21, 2024
One expert views Amtagvi’s approval as a catalyst for further investment in TIL therapies, akin to how Kymriah’s 2017 clearance buoyed CAR-T treatment.
PLOS: DNA Science
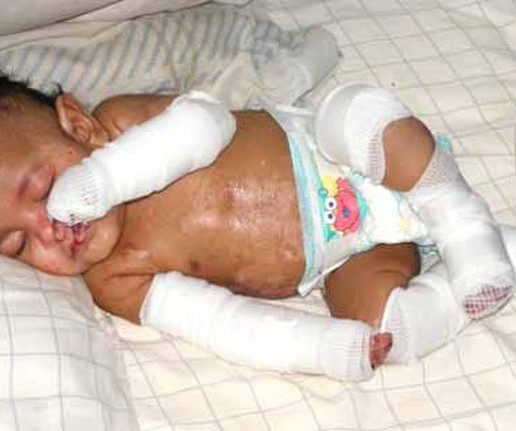
JUNE 1, 2023
The newest FDA-approved gene therapy treats the severe, skin-peeling condition dystrophic epidermolysis bullosa (DEB). The gene treatment has been a long time coming, but it differs from the handful of other approved gene therapies: it isn’t a one-and-done.

Drugs.com
JUNE 23, 2023
Food and Drug Administration on Thursday approved the drug Elevidys, the first gene therapy for the treatment of children with Duchenne muscular dystrophy (DMD). FRIDAY, June 23, 2023 -- The U.S. The groundbreaking treatment will not be cheap:

BioPharma Drive: Drug Pricing
JUNE 29, 2023
After a prolonged journey, the medicine, known as Roctavian, is now cleared for certain patients with hemophilia A, the more common form of the rare bleeding disorder.

Drugs.com
JUNE 23, 2023
Food and Drug Administration on Thursday approved the drug Elevidys, the first gene therapy for the treatment of children with Duchenne muscular dystrophy (DMD). FRIDAY, June 23, 2023 -- The U.S. The groundbreaking treatment will not be cheap:

BioPharma Drive: Drug Pricing
MARCH 19, 2024
clearance of Lenmeldy, for a rare and inherited metabolic disease, triggers an additional payout related to Kyowa Kirin’s recent deal to acquire the once high-flying gene therapy developer.
Broad Institute
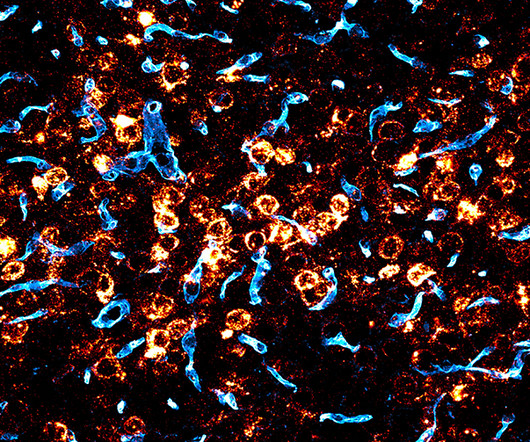
MAY 16, 2024
Gene therapy could potentially treat a range of severe genetic brain disorders, which currently have no cures and few treatment options. Since we came to the Broad we’ve been focused on the mission of enabling gene therapies for the central nervous system,” said Deverman, senior author on the study. “If

Drugs.com
JUNE 30, 2023
Food and Drug Administration on Thursday approved a costly single-dose gene therapy for patients with severe hemophilia A. FRIDAY, June 30, 2023 -- The U.S. The treatment, Roctavian (valoctocogene roxaparvovec), will cost $2.9 million for a single.
The Pharma Data
DECEMBER 21, 2020
FDA Approves First Oral Hormone Therapy for Advanced Prostate Cancer. 21, 2020 — Orgovyx (relugolix) is now approved to treat advanced prostate cancer and is the first oral hormone therapy approved for this indication, the U.S. Professional. MONDAY, Dec. Food and Drug Administration announced Friday.

The Pharma Data
MAY 27, 2022
Novartis today announced the US Food and Drug Administration (FDA) has granted accelerated approval for Kymriah ® (tisagenlecleucel) for the treatment of adult patients with relapsed or refractory (r/r) follicular lymphoma (FL) after two or more lines of systemic therapy.
BioPharma Drive: Drug Pricing
AUGUST 14, 2023
Elrexfio is the third bispecific antibody cleared to treat the blood cancer, and will compete with other therapies that target the “BCMA” protein on myeloma cells.

Drugs.com
FEBRUARY 19, 2024
Food and Drug Administration has approved a novel treatment for advanced melanoma, the most deadly form of skin cancer. becomes the first cellular therapy approved to. Amtagvi, made by Iovance Biotherapeutics Inc.,
The Pharma Data
AUGUST 9, 2021
deputy director of the Office of Rare Diseases, Pediatrics, Urologic and Reproductive Medicine in the FDA’s Center for Drug Evaluation and Research. “Today’s approval brings patients with Pompe disease another enzyme replacement therapy option for this rare disease.

The Pharma Data
APRIL 19, 2022
Kite’s Global CAR T-Cell Therapy Manufacturing Network Increasing Capacity by 50% to Meet Patient Demand for New Cancer Therapies. — Scalable and Adaptable Facility Provides Flexibility for Current and Future Cell Therapy Innovation. The site will produce Kite’s FDA approved CAR T-cell therapy used to treat blood cancer.

Drugs.com
FEBRUARY 19, 2024
Food and Drug Administration has approved a novel treatment for advanced melanoma, the most deadly form of skin cancer.Amtagvi, made by Iovance Biotherapeutics Inc., becomes the first cellular therapy approved to.
BioPharma Drive: Drug Pricing
JANUARY 5, 2024
recently submitted its MDMA capsules for FDA approval, following two positive studies. Lykos Therapeutics, formerly known as MAPS Public Benefit Corp.,

The Pharma Data
AUGUST 19, 2021
Food and Drug Administration (FDA) approval of the VENTANA MMR RxDx Panel, advancing the company’s commitment to personalised healthcare through tests that determine which patients are most likely to benefit from specific and targeted therapies. today announced U.S. A bout the VENTANA MMR RxDx Panel.

BioPharma Drive: Drug Pricing
JUNE 30, 2023
The agency granted a long-awaited clearance on Thursday, but unexpected aspects of the hemophilia treatment’s label had some investors worried about its commercial prospects.

The Pharma Data
DECEMBER 17, 2020
Food and Drug Administration (FDA) had several approvals this week. Food and Drug Administration (FDA) has approved belimumab (Benlysta®), the first-ever treatment for adults with lupus nephritis (LN) who are currently receiving standard therapy. The FDA approval of Klisyri is a significant milestone for Athenex.

FDA Law Blog: Drug Discovery
DECEMBER 7, 2022
Valentine — On November 22, 2022, FDA approved CSL Behring’s BLA for Hemgenix (etranacogene dezaparvovec), an AAV-based gene therapy for the treatment of adults with Hemophilia B who currently use Factor IX prophylaxis therapy, have current or historical life-threatening hemorrhage, or have repeated, serious spontaneous bleeding episodes.

The Pharma Data
JANUARY 18, 2021
“It’s important that Impulse Dynamics acted quickly in a joint effort with the FDA to remove this potential barrier standing in the way of critical diagnostic procedures for a subset of patients with HF. CCM therapy is the first approach designed to optimize heart contraction, allowing more oxygen-rich blood to reach the body.

Fierce BioTech
OCTOBER 5, 2023
Discover Strategies for Combatting Disruptions in Gene Therapy Development Cell and gene therapy development has exploded, with Q4 2022 showing more FDA approvals than over the past five years combined.[1]

ASPET
JANUARY 31, 2024
Despite the standard of care therapy which includes surgical resection, temozolomide chemotherapy, radiation and the newly added tumor treating fields, median survival remains only ~20 months. Glioblastoma (GBM) is the most frequently diagnosed primary CNS tumor in adults.

Drugs.com
JUNE 30, 2023
Food and Drug Administration on Thursday approved a costly single-dose gene therapy for patients with severe hemophilia A, a life-threatening hereditary bleeding disorder. FRIDAY, June 30, 2023 -- The U.S. The treatment is not cheap: Roctavian will.

The Pharma Data
JANUARY 17, 2021
Food and Drug Administration (FDA) approved Janssen Pharmaceuticals ’ (a Johnson and Johnson company) Darzalex Faspro for adults with newly diagnosed light chain amyloidosis. It was approved in combination with bortezomib, cyclophosphamide and dexamethasone (D-VCd). Michael Vi/Shutterstock. It was developed with Genmab.
The Pharma Data
AUGUST 24, 2020
The FDA has approved a request from American Gene Technologies to begin a clinical study into its HIV gene therapy. In a paper published in Molecular Therapy: Methods & Clinical Development , the team discussed why they choose this type of gene therapy to treat HIV. Air Force photo by Kemberly Groue.

Chemical Biology and Drug Design
OCTOBER 10, 2023
Later, a new type of non-nucleoside reverse transcriptase inhibitors (NNRTIs) were approved as anti-HIV drugs. Zidovudine, didanosine, and stavudine are FDA-approved NRTIs, while nevirapine, efavirenz, and delavirdine are FDA-approved NNRTIs.

The Pharma Data
JANUARY 21, 2021
Food and Drug Administration (FDA) gave a greenlight for ViiV Healthcare ’s Cabenuva. Today’s FDA approval of Cabenuva represents a shift in the way HIV is treated, offering people living with HIV a completely new approach to care,” said Lynn Baxter, Head of North America, ViiV Healthcare. clinical practices. “Not

The Pharma Data
SEPTEMBER 7, 2020
Genentech’s once-daily oral therapy Gavreto (pralsetinib) has secured FDA backing in the treatment of metastatic rearranged during transfection (RET) fusion-positive non-small cell lung cancer (NSCLC), it has emerged. In 27 treatment-naïve NSCLC patients, the ORR was found to be 70% and CRR was 11%. Matt Fellows. Source link.
BioPharma Drive: Drug Pricing
SEPTEMBER 5, 2023
The FDA-approved therapy, called Palforzia, was little used, leading Nestle to abandon a business it had secured in a $2.6 billion deal three years ago.

The Pharma Data
JUNE 22, 2023
FDA approval Pfizer (NYSE: PFE) announced that the U.S. 3,4,5,6) “Despite treatment advancement in metastatic castration-resistant prostate cancer, the disease can progress quickly, and many patients may only receive one line of therapy. Pfizer’s TALZENNA® in combination with XTANDI® receives U.S.

The Premier Consulting Blog
NOVEMBER 12, 2024
For drug developers the 505(b)(2) pathway presents an expedited pathway to FDA approval. In our experience, the conversation on 505(b)(2) pathways commonly focuses on predicate data or bridging strategies to accelerate FDA approval but lacks dialogue on the needs of US payers. This is standard formulary policy language.

The Pharma Data
AUGUST 31, 2021
today announced a label update for KEYTRUDA, Merck’s anti-PD-1 therapy, for its indication in first-line advanced urothelial carcinoma (bladder cancer) in the U.S. Food and Drug Administration (FDA) has converted this indication from an accelerated to a full (regular) approval. Source link: [link].
The Pharma Data
JANUARY 22, 2021
This approval will allow some patients the option of receiving once-monthly injections in lieu of a daily oral treatment regimen,” said Dr. John Farley, director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research.

The Pharma Data
MAY 19, 2023
ABBOTT RECEIVES FDA APPROVAL FOR TACTIFLEX™ ABLATION CATHETER FOR TREATMENT OF ABNORMAL HEART RHYTHM Abbott (NYSE: ABT) today announced that the U.S. 6 “Abbott is leading the way in helping doctors manage common arrhythmias with the most holistic portfolio for this condition in the world,” said Christopher Piorkowski, M.D.,

The Pharma Data
AUGUST 27, 2020
Roche is committed to improve patient outcomes by providing multiple testing options that support decision-making during all lines of therapy. Food and Drug Administration (FDA) has approved FoundationOne®Liquid CDx, Foundation Medicine’s comprehensive pan-tumour liquid biopsy test for patients with solid tumours.

The Pharma Data
OCTOBER 8, 2021
Rethymic is the first thymus tissue product approved in the U.S. “Today’s action marks the first FDA approval of a therapy to treat this very rare and devastating disease in children,” said Peter Marks, M.D., director of the FDA’s Center for Biologics Evaluation and Research.

The Pharma Data
NOVEMBER 29, 2021
Conventional treatment for ovarian cancer includes surgery to remove as many of the tumors as possible, chemotherapy to stop the growth of malignant cells or other targeted therapy to identify and attack specific cancer cells. The FDA previously granted Cytalux orphan-drug , priority and fast track designations. Related Information.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content