FDA Returns Disappointing News for ALS Stem Cell Therapy
PLOS: DNA Science
OCTOBER 19, 2023
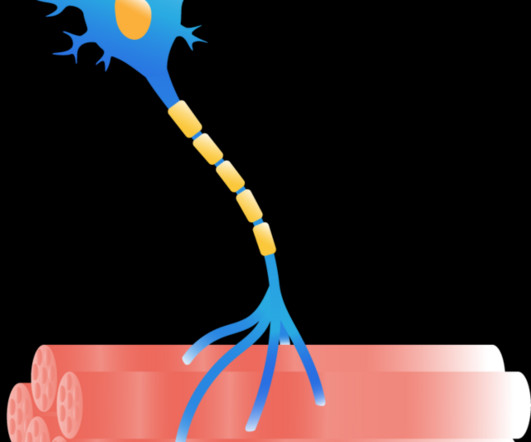
Last week DNA Science covered a setback in a clinical trial of a gene therapy for Duchenne muscular dystrophy (DMD). Also recently, FDA’s Cellular, Tissue, and Gene Therapies Advisory Committe turned down a stem cell treatment for amyotrophic lateral sclerosis, aka ALS, Lou Gehrig’s disease, or motor neuron disease.









































Let's personalize your content