Keeping tabs on Covid-19: UK firm tests oral delivery of Covid-19 vaccine and Novavax aims to distribute 51 million vaccines to Australia
The Pharma Data
JANUARY 22, 2021
As research developments into RNA vaccines help scientists accelerate drug candidates to arm the immune system against coronavirus, Pharma IQ ’s Keeping tabs on Covid-19 update returns with news from some of the biotechnology innovators leading the fight against the global pandemic.


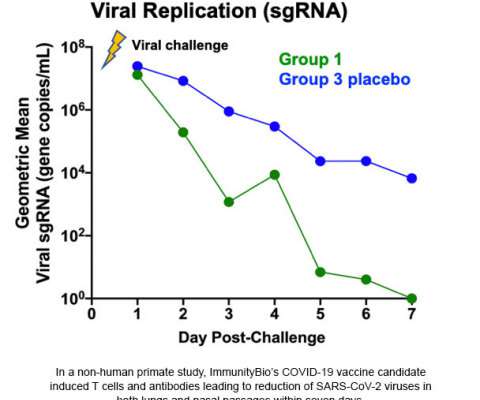













Let's personalize your content