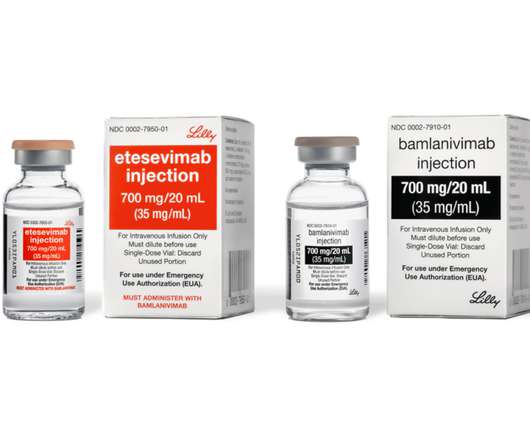
bamlanivimab and etesevimab together for treatment of COVID-19 in the U.S.
The Pharma Data
APRIL 16, 2021
1.429 California strain that currently accounts for 50 percent of the virus in California and over 10 percent across a number of additional states. Due to the rapidly evolving and geographically diverse nature of the SARS-CoV-2 virus, continued scientific innovation remains critical to develop additional treatments. In the U.S.,














Let's personalize your content