Leveraging agonist antibodies to address immunological diseases
Drug Target Review
NOVEMBER 7, 2024
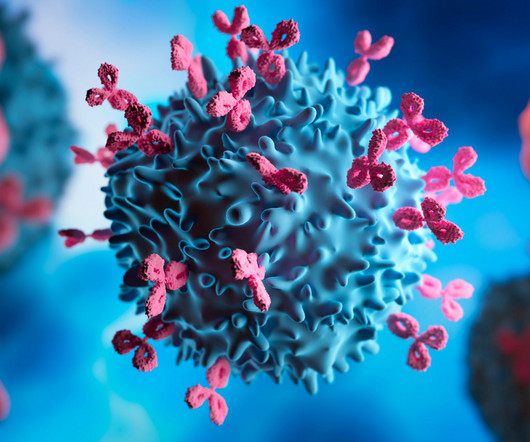
Agonist antibodies of immune checkpoint regulators These represent a groundbreaking class of immunotherapeutic agents that mimic the natural function of endogenous ligands by binding to specific cell-surface receptors. In these conditions, the goal of therapy is typically to suppress or mitigate immune activity.







































Let's personalize your content