Second-Generation mRNA COVID-19 Vaccine Candidate, CV2CoV, Demonstrates Improved Immune Response and Protection in Preclinical Study
The Pharma Data
AUGUST 16, 2021
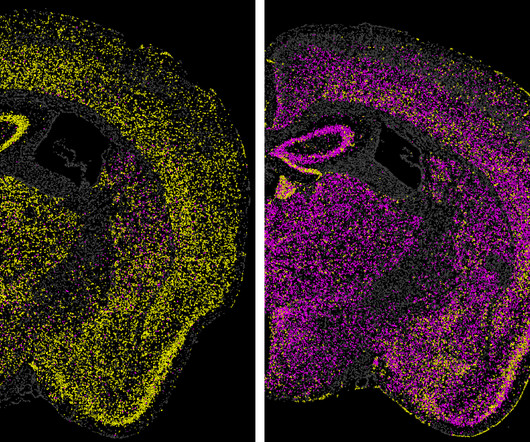
Better activation of innate and adaptive immune responses was achieved with CV2CoV, resulting in faster response onset, higher titers of antibodies, and stronger memory B and T cell activation as compared to the first-generation candidate, CVnCoV. “In Induction of innate immunity was investigated via specific cytokine markers.














Let's personalize your content