Chlamydia Vaccine Shows Promise in Early Trial
Drugs.com
APRIL 12, 2024
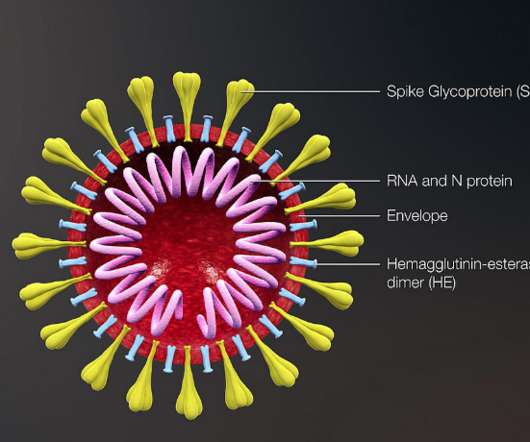
FRIDAY, April 12, 2024 -- A chlamydia vaccine has triggered immune responses in an early trial, raising hopes that one day it might help curb the spread of the sexually transmitted infection (STI).There There is currently no vaccine for chlamydia, which.


































Let's personalize your content