Gene editing extends lifespan in mouse model of prion disease
Broad Institute
JANUARY 14, 2025
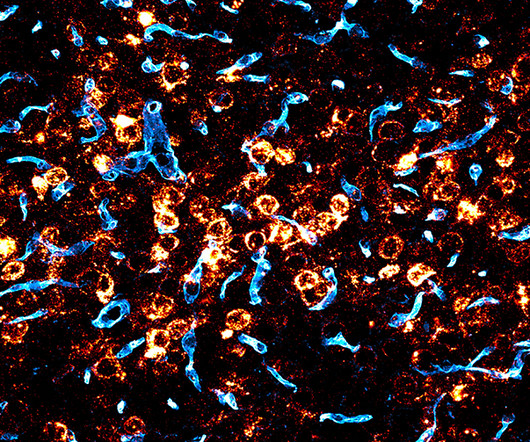
We are hopeful the results might inform the future development of a one-time treatment for this important class of diseases. We realized it was this golden opportunity to use human genetics to inform base editing, Minikel said. But the researchers needed to deliver the base editors to the brain.













































Let's personalize your content