The Next Generation of Vaccine Mega Trials
PPD
JUNE 24, 2024
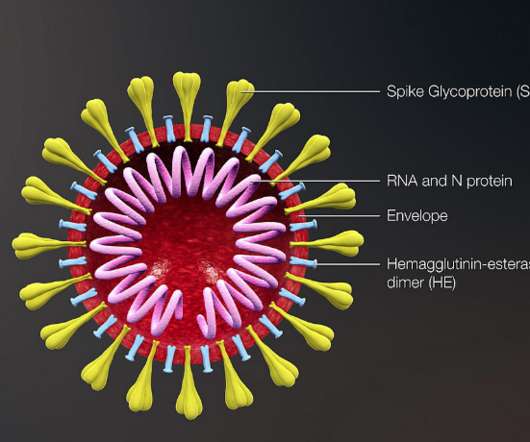
In the realm of vaccine development, mega trials — studies enrolling 5,000 subjects or more — have been instrumental in the fight against many pathogens, including influenza, rotavirus, malaria, RSV and most recently in the rapid development of vaccines against COVID-19.




































Let's personalize your content