This week in drug discovery (29 January – 2 February)
Drug Discovery World
FEBRUARY 2, 2024
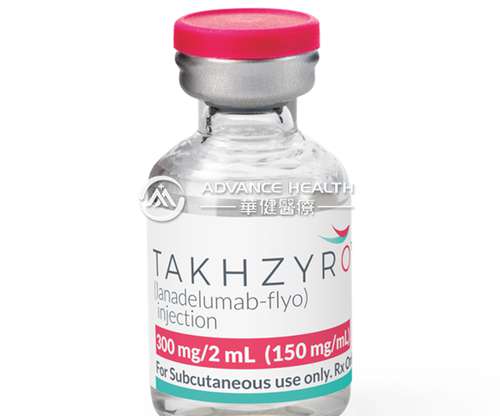
Sunday 4 February is World Cancer Day, an international awareness day led by the Union for International Cancer Control (UICC), so the round-up this week will focus on oncology, including the UK approval of a rare blood cancer drug and a novel production process for CAR-T therapies.









































Let's personalize your content